As Noxa was first identified as a p53 target gene, the stabilization and activation of p53 would have been an attractive possibility for BAY 73-4506 apoptosis induction by PIs. However, PI-mediated tumor cell killing was also observed in p53-deficient cells and independently of NF-kB inhibition suggesting that other signaling pathways targeted by the proteasome are even more crucial for cell death induction by PIs. One of those might be instigated by members of the mitogen-activated protein kinase family, the c-Jun N-terminal kinases that were reproducibly found to be activated in PI-treated cells. More intriguingly, inhibition of JNK activity by either dominant-negative JNKs or by RNA interference rendered the cells resistant toward cell death 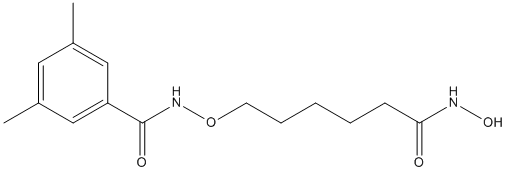 induction by PIs. Thus, it appears that JNKs, in addition to several other pathways in which they were shown to contribute to apoptosis signaling, are also crucial players in PI-induced apoptosis. Three JNK isoforms with different splice variants are expressed either ubiquitously or preferentially in neuronal and heart tissues. They were originally identified by their ability to specifically phosphorylate and activate c-Jun, a constituent of the activator protein-1 transcription factor that is involved in the increased expression of several pro-apoptotic genes such as TNF-a, Fasligand, Bak and Bim. Although silencing of the c-Jun/AP-1 pathway conferred resistance to JNK-mediated apoptosis in several cellular systems, the observed stimulus- and cell typedependent manner of protection suggests participation of other downstream effectors. Indeed, JNKs appear to control apoptosis in quite a versatile manner as they not only phosphorylate and activate other pro-apoptotic transcriptions factors including p53 and c-Myc, but also several Bcl-2 family proteins causing inhibition of pro-survival members such as Bcl-2, Bcl-XL and Mcl-1 and activation of pro-apoptotic members such as Bim and Bad. However, although these phosphorylation events are consistent with the observation that JNKs are required for stress-induced activation of the mitochondrial death pathway, their contributions to apoptosis are controversially discussed. In addition, it is unknown whether JNK1 and JNK2 exhibit redundant or specific functions in PI-induced apoptosis and whether they are involved in the induction of Noxa. To elucidate these questions in more detail, we compared PIinduced apoptosis signaling of immortalized mouse embryonic fibroblasts that differ in their JNK1 and/or JNK2 status. In addition to our findings that JNK1 greatly accelerates de novo expression of Noxa and subsequent apoptosis, we also observed that both processes were strongly impaired in the presence of JNK2 implying oppositional roles for these isoforms in PI-induced apoptosis. Inhibition of the proteasome either on its own or in combination with other apoptotic stimuli is a Nutlin-3 powerful means to specifically eradicate tumor cells, but the underlying molecular pathways are only incompletely deciphered. JNKs and the BH3-only protein Noxa were reproducibly demonstrated in many diverse systems to be essential constituents of this process as inhibition of their activity and/or expression substantially protected cells from PI-induced apoptosis. However, these two pathways have never been connected before and the contributions of individual JNK isoforms to PI-mediated induction of Noxa and apoptosis were completely unknown.
induction by PIs. Thus, it appears that JNKs, in addition to several other pathways in which they were shown to contribute to apoptosis signaling, are also crucial players in PI-induced apoptosis. Three JNK isoforms with different splice variants are expressed either ubiquitously or preferentially in neuronal and heart tissues. They were originally identified by their ability to specifically phosphorylate and activate c-Jun, a constituent of the activator protein-1 transcription factor that is involved in the increased expression of several pro-apoptotic genes such as TNF-a, Fasligand, Bak and Bim. Although silencing of the c-Jun/AP-1 pathway conferred resistance to JNK-mediated apoptosis in several cellular systems, the observed stimulus- and cell typedependent manner of protection suggests participation of other downstream effectors. Indeed, JNKs appear to control apoptosis in quite a versatile manner as they not only phosphorylate and activate other pro-apoptotic transcriptions factors including p53 and c-Myc, but also several Bcl-2 family proteins causing inhibition of pro-survival members such as Bcl-2, Bcl-XL and Mcl-1 and activation of pro-apoptotic members such as Bim and Bad. However, although these phosphorylation events are consistent with the observation that JNKs are required for stress-induced activation of the mitochondrial death pathway, their contributions to apoptosis are controversially discussed. In addition, it is unknown whether JNK1 and JNK2 exhibit redundant or specific functions in PI-induced apoptosis and whether they are involved in the induction of Noxa. To elucidate these questions in more detail, we compared PIinduced apoptosis signaling of immortalized mouse embryonic fibroblasts that differ in their JNK1 and/or JNK2 status. In addition to our findings that JNK1 greatly accelerates de novo expression of Noxa and subsequent apoptosis, we also observed that both processes were strongly impaired in the presence of JNK2 implying oppositional roles for these isoforms in PI-induced apoptosis. Inhibition of the proteasome either on its own or in combination with other apoptotic stimuli is a Nutlin-3 powerful means to specifically eradicate tumor cells, but the underlying molecular pathways are only incompletely deciphered. JNKs and the BH3-only protein Noxa were reproducibly demonstrated in many diverse systems to be essential constituents of this process as inhibition of their activity and/or expression substantially protected cells from PI-induced apoptosis. However, these two pathways have never been connected before and the contributions of individual JNK isoforms to PI-mediated induction of Noxa and apoptosis were completely unknown.
Monthly Archives: August 2019
The development of orally bioavailable PIs with distinct mode of action is a possible way to circumvent these issues
We suspect dPRL-1/dPRL-1NC may physically interfere with either Src or an effector of Src function. While dPRL-1s ability to inhibit growth is in concordance with one report from the mammalian literature, there are certainly differences to highlight Nutlin-3 between Drosophila and mammalian studies. Sequence analysis shows that the aspartate, that serves as a proton donor is present in Drosophila but not in the context of the WPD loop, as seen in mammalian PRL family members. While this aspartate is also not found in WPD loop in other PTPs like VHR, cdc14, and PTEN, it may point to different substrates between mammals and flies. In addition, catalytic activity of mammalian PRL1 is regulated by the redox environment,,, and thought to exist in an inactive conformation under normal cellular conditions. Possibly, differences in redox AG-013736 319460-85-0 regulation between Drosophila and cultured mammalian cells could account for differing outcomes in response to PRL-1 overexpression. For example, altered redox environments in transformed cells could switch PRLs to an abnormal, catalytically active state. Another important difference between Drosophila and mammals may be the p53 network. While supporting the model that PRL-3 is a transcriptional target of p53, Min et al., report that PRL-3 then functions in 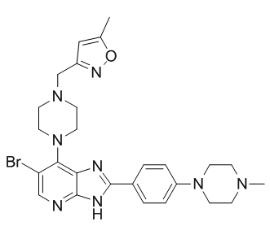 a negative, autoregulatory loop by decreasing levels of p53, which would help transform cells. They identify MDM2 and PIRH2 as the important players in this pathway; but since neither protein is found in Drosophila, this oncogenic path is not conserved. In spite of the differences between mammals and Drosophila, flies have successfully informed numerous mechanisms that contribute to human cancer biology. We have established a new system that has revealed novel characteristics of the PRL family and will help decipher the role PRLs play in cancers. The paradigm of cancer treatment has been dramatically changed by the introduction of small molecular compounds that target the “Achilles’heel” of cancer cells. The proteasome is a proteolytic machinery that executes the degradation of polyubiquitinated proteins to maintain cellular homeostasis. Cancer cells are very sensitive to proteotoxic stress because of intracellular protein overload due to rapid cell cycling and apoptosis inhibition. This feature makes proteasome inhibition a unique and effective way to kill cancer cells that can tolerate conventional therapies. Bortezomib is the first proteasome inhibitor approved for clinical application, which preferentially targets ?1 and ?5 subunits of the proteasome. This drug is particularly effective for multiple myeloma, because it accelerates the unfolded protein response via down-regulation of histone deacetylases and targets cell adhesion-mediated drug resistance via down-regulation of very late antigen-4. Accordingly, bortezomib is now indispensable for the treatment of MM in combination with other anti-cancer drugs including alkylating agents, corticosteroids and HDAC inhibitors. Although bortezomib therapy is a major advance in clinical oncology, there are at least three major problems to be resolved as soon as possible. First, bortezomib has several possible off-target toxicities. Second, the development of intrinsic and acquired resistance to bortezomib is an emerging problem. Third, bortezomib should be administered intravenously on biweekly schedules with treatment periods extending for 6 months or more.
a negative, autoregulatory loop by decreasing levels of p53, which would help transform cells. They identify MDM2 and PIRH2 as the important players in this pathway; but since neither protein is found in Drosophila, this oncogenic path is not conserved. In spite of the differences between mammals and Drosophila, flies have successfully informed numerous mechanisms that contribute to human cancer biology. We have established a new system that has revealed novel characteristics of the PRL family and will help decipher the role PRLs play in cancers. The paradigm of cancer treatment has been dramatically changed by the introduction of small molecular compounds that target the “Achilles’heel” of cancer cells. The proteasome is a proteolytic machinery that executes the degradation of polyubiquitinated proteins to maintain cellular homeostasis. Cancer cells are very sensitive to proteotoxic stress because of intracellular protein overload due to rapid cell cycling and apoptosis inhibition. This feature makes proteasome inhibition a unique and effective way to kill cancer cells that can tolerate conventional therapies. Bortezomib is the first proteasome inhibitor approved for clinical application, which preferentially targets ?1 and ?5 subunits of the proteasome. This drug is particularly effective for multiple myeloma, because it accelerates the unfolded protein response via down-regulation of histone deacetylases and targets cell adhesion-mediated drug resistance via down-regulation of very late antigen-4. Accordingly, bortezomib is now indispensable for the treatment of MM in combination with other anti-cancer drugs including alkylating agents, corticosteroids and HDAC inhibitors. Although bortezomib therapy is a major advance in clinical oncology, there are at least three major problems to be resolved as soon as possible. First, bortezomib has several possible off-target toxicities. Second, the development of intrinsic and acquired resistance to bortezomib is an emerging problem. Third, bortezomib should be administered intravenously on biweekly schedules with treatment periods extending for 6 months or more.
The current de novo drug discovery and development paradigm is ineffective for dealing with biological threat agents
MMP-3 serum levels than patients without dCVS. Given an increased permeability of the blood-brain barrier in patients with SAH this elevation may be caused by LY294002 astrocytic MMP-3 production in our study population correlating with the time at highest risk for CVS. In contrast to the early up-regulation of MMP-9 in SAH patients compared to healthy controls, MMP-3 remained continuously lower. MMP-3 is known to activate MMP-9. Therefore, one possible explanation for the observed imbalance of MMP-3 and -9 might be a counterregulatory decrease of MMP3 in response to overactivity of MMP-9. Similar dynamics have been described in patients suffering from ischemic stroke. Notably, MMP-3 has been shown to be linked to coagulation. Therefore, the sharp decrease of MMP-3 levels during the first days after SAH might be attributable to increased consumption of coagulatory factors, triggered by aneurysm rupture. The expression of MMPs under physiologic conditions is mainly controlled at the transcriptional level. A tight balance between MMP activity and their endogenous tissue inhibitors is crucial. TIMP-1 is highly inhibitory for MMP-9. We found a delayed increase of TIMP-1 in SAH patients starting on day 6 indicating an overshoot of MMP activity during the initial phase 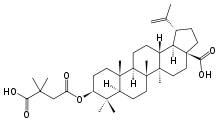 after SAH. This is the first study that investigates TIMP-1 serum concentrations in SAH patients so far. However, animal studies suggest an upregulation of TIMP-1 after experimental SAH. In line with the delayed increase of TIMP-1, TIMP-3 was significantly reduced in the acute phase after SAH. Since TIMP-3 has been shown to have anti-inflammatory effects this downregulation might corroborate the importance of pro-inflammatory mechanisms in the pathophysiology of SAH in general and for early brain injury in particular. Our study, designed as a pilot study, only included a small number of patients, a potentially limiting factor. Importantly, the statistical models were corrected for important covariates and patients were well matched and showed a representative distribution of SJN 2511 ALK inhibitor demographic and clinical characteristics. In addition the incidence of CVS, DCI and mortality was similar to previously local and international published data. However due to the low sample size our results will have to be verified in a larger patient sample. To our knowledge this is the first longitudinal study focusing on MMPs and their respective tissue inhibitors in SAH. Early changes of MMPs and their tissue inhibitors were observed, they might play a role in the pathophysiology of acute SAH. These high-priority bioterrorism agents are defined by their ability to be easily disseminated or transmitted, their high mortality rates or capacity to generate major public health impacts, their potential for causing mass panic and social disruption, and the requirement for government action to ensure public preparedness. Moreover, there is a paucity of FDA-approved therapeutic options for the bacterial agents and no approved therapeutics for the viral pathogens. The threat of these biological agents is exacerbated by the incessant risk that these agents could become resistant to current therapeutic agents by conventional as well as genetic means. In addition, there is no effective way to address the threats of emerging, engineered, or advanced pathogens in a timely manner, as the current drug discovery and development paradigm takes up to 20 years for introduction of a new, approved drug into the market.
after SAH. This is the first study that investigates TIMP-1 serum concentrations in SAH patients so far. However, animal studies suggest an upregulation of TIMP-1 after experimental SAH. In line with the delayed increase of TIMP-1, TIMP-3 was significantly reduced in the acute phase after SAH. Since TIMP-3 has been shown to have anti-inflammatory effects this downregulation might corroborate the importance of pro-inflammatory mechanisms in the pathophysiology of SAH in general and for early brain injury in particular. Our study, designed as a pilot study, only included a small number of patients, a potentially limiting factor. Importantly, the statistical models were corrected for important covariates and patients were well matched and showed a representative distribution of SJN 2511 ALK inhibitor demographic and clinical characteristics. In addition the incidence of CVS, DCI and mortality was similar to previously local and international published data. However due to the low sample size our results will have to be verified in a larger patient sample. To our knowledge this is the first longitudinal study focusing on MMPs and their respective tissue inhibitors in SAH. Early changes of MMPs and their tissue inhibitors were observed, they might play a role in the pathophysiology of acute SAH. These high-priority bioterrorism agents are defined by their ability to be easily disseminated or transmitted, their high mortality rates or capacity to generate major public health impacts, their potential for causing mass panic and social disruption, and the requirement for government action to ensure public preparedness. Moreover, there is a paucity of FDA-approved therapeutic options for the bacterial agents and no approved therapeutics for the viral pathogens. The threat of these biological agents is exacerbated by the incessant risk that these agents could become resistant to current therapeutic agents by conventional as well as genetic means. In addition, there is no effective way to address the threats of emerging, engineered, or advanced pathogens in a timely manner, as the current drug discovery and development paradigm takes up to 20 years for introduction of a new, approved drug into the market.