This model implies ING1 as a prognostic factor for response to targeted cancer therapy: patients with tumors expressing low ING1-levels might not significantly profit from certain proteasome-inhibitors or other anticancer agents, that involve the induction of p53-dependent apoptosis in the CHIR-99021 252917-06-9 selective killing of tumor cells. Metformin is an oral insulin-sensitizing agent commonly used either alone or in combination with other antihyperglycemic drugs in patients with type 2 diabetes. Based on various population-based analyses, prescription of metformin in patients with type 2 diabetes increased by about 50% in European countries. The glucose-lowering effect of metformin is largely attributable to inhibition of hepatic gluconeogenesis, and additionally, insulinstimulated glucose uptake into skeletal muscle cells and adipocytes is increased by metformin. Recently, it has been shown that organic cation transporters are crucial for the uptake of metformin and these membrane transport proteins are expressed at significant levels in metformin target tissues such as liver, muscle, and adipose tissue. Data from OCT1 knockout mice as well as from healthy volunteers carrying OCT1 variants clearly indicate an alteration of metformin disposition and subsequent consequences for plasma glucose levels. Since metformin does not undergo hepatic metabolism, drug-drug interaction by inhibition of OCT transporters might be important. Because OCT1 is expressed in human liver, alteration of hepatic metformin uptake may be assumed, thereby resulting in poor response to metformin treatment due to reduced glucose-lowering effects. Otherwise, drug-drug interaction with OCT2, which is expressed in proximal tubule epithelial cells, would probably increase systemic disposition of metformin by reduced renal clearance. Recently, a strong inhibiting effect of repaglinide and rosiglitazone on OCT1-mediated metformin transport as well as of several drugs on OCT2-mediated metformin transport in vitro has been reported. Clinically, concomitant use of the potent OCT2 inhibitors cimetidine and verapamil in cisplatin-treated patients resulted in a lower risk for cisplatin-related nephrotoxicity since the antitumor drug cisplatin is an OCT2 substrate. This clinical observation is supported by animal data, clearly demonstrating that cimetidinerelated inhibition of the OCT2 transporter alters cisplatin uptake in the kidney. These examples suggest that OCT-mediated drug-drug interactions appear to be clinically relevant. Hundreds of xenobiotics including drugs potentially inhibiting OCTs were tested in the past and several new inhibitors have been identified. However, systematic data regarding the important drug class of proton 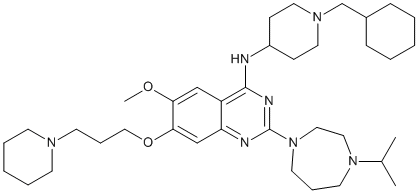 pump inhibitors are still missing although PPIs are frequently used in metformin-treated patients with metabolic syndrome and cardiovascular diseases. Moreover, gastroesophageal reflux disease is commonly seen in patients with type 2 diabetes and PPIs are the drugs of best choice in treatment of GERD. With the recent advances in the understanding of the role of drug transporters in pharmacokinetics it has become critical to elucidate drug-drug interactions that are rooted in transporters. Drug transporters can be generally classified as either uptake or MDV3100 efflux transporters characterizing whether they facilitate drug entry into a cell or efflux out of a cell. In the present paper we focused on the uptake transporter proteins OCT1, OCT2, and OCT3 since the antidiabetic drug metformin is a substrate for each and there is already evidence.
pump inhibitors are still missing although PPIs are frequently used in metformin-treated patients with metabolic syndrome and cardiovascular diseases. Moreover, gastroesophageal reflux disease is commonly seen in patients with type 2 diabetes and PPIs are the drugs of best choice in treatment of GERD. With the recent advances in the understanding of the role of drug transporters in pharmacokinetics it has become critical to elucidate drug-drug interactions that are rooted in transporters. Drug transporters can be generally classified as either uptake or MDV3100 efflux transporters characterizing whether they facilitate drug entry into a cell or efflux out of a cell. In the present paper we focused on the uptake transporter proteins OCT1, OCT2, and OCT3 since the antidiabetic drug metformin is a substrate for each and there is already evidence.
Monthly Archives: August 2019
It is conceivable that larger substrates utilize this exosite as a point of leverage for larger substrates
These findings lend fresh support to the longstanding prediction that IDE inhibitors could hold therapeutic potential as primary or adjunct treatments for diabetes. Here we describe the rational design, synthesis, enzymologic characterization, and co-crystallographic analysis of potent and Fulvestrant selective peptide hydroxamate inhibitors of IDE. In addition, we use these compounds to show that IDE regulates fundamental aspects of insulin catabolism and signaling in a manner that implies that IDE inhibitors could have anti-diabetic properties. Although the inhibitors described in this study are unlikely to have immediate value as therapeutic agents due to their peptidic nature, their development and the chemical biology they make possible are significant in several important respects. First and foremost, these compounds constitute the first potent and selective inhibitors of IDE or, indeed, of any member of the inverzincin superfamily of zinc-metalloproteases. Given the longstanding interest in IDE in general, and the predicted therapeutic value of IDE inhibitors in particular, why has their development proved so elusive for so long? The answer can be traced to the distinctive structure of IDE��s active site, which in turn reflects the separate evolutionary origins of this protease superfamily. As documented by earlier studies and the present work, IDE��s active site is bipartite, consisting of two distinct domains contained within the C- and N-terminal halves of the protease. The active site becomes fully formed only when the protease is in the closed conformation, and it is disrupted completely upon transition to the open conformation. These very large conformational changes occurring during the catalytic cycle of IDE essentially render its active site a “moving GSI-IX target,” one that cannot easily be stably occupied by small molecules, even those containing a strong zinc-binding moiety. As our co-crystal structure reveals, the potency of Ii1 can be traced to its unique ability to interact simultaneously with both the N- and C-terminal portions of the active site. In so doing, Ii1 appears to “lock” the protease in the closed, inactive conformation-a feature that is likely to be indispensable for effective IDE inhibitors. Second, these IDE inhibitors grant several new insights into the enzymology of this poorly understood protease. A particularly puzzling property is the substrate-dependence of Ki values for inhibition of IDE by Ii1, wherein smaller substrates show lower Ki values than larger substrates. These two categories of substrate have in fact been shown to exhibit strikingly different behaviors in multiple contexts. For example, the hydrolysis of short substrates-but not longer ones-can be profoundly activated by ATP and other nucleotide polyphosphates, inorganic triphosphate, as well as by structurally unrelated drug-like molecules. 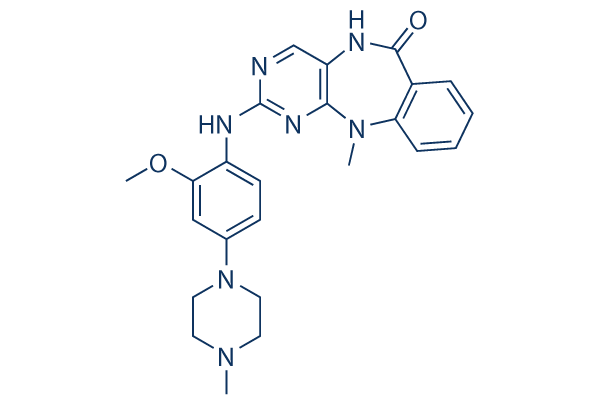 In terms of the differences in Ki values, we speculate that larger substrates may be more capable than smaller ones of effecting the transition between the closed and open configurations, resulting in an increased off rate for the inhibitor. It may also be that the inhibitor can be trapped inside the internal chamber only in the case of smaller substrates. Alternatively, given that 2 residues within Ii1 protrude into the internal chamber, it may be that larger substrates sterically block a subset of binding modes of the inhibitor. In this context, it is relevant to note that larger substrates are known to interact with an exosite present inside the catalytic chamber but opposite to the active site.
In terms of the differences in Ki values, we speculate that larger substrates may be more capable than smaller ones of effecting the transition between the closed and open configurations, resulting in an increased off rate for the inhibitor. It may also be that the inhibitor can be trapped inside the internal chamber only in the case of smaller substrates. Alternatively, given that 2 residues within Ii1 protrude into the internal chamber, it may be that larger substrates sterically block a subset of binding modes of the inhibitor. In this context, it is relevant to note that larger substrates are known to interact with an exosite present inside the catalytic chamber but opposite to the active site.
The earlier study demonstrated that knockdown of TRPM7 dramatically reduced neuronal cell death induced by oxygen glucose deprivation
In addition, the channel also contributes to ischemic brain pathology. It has been shown that TRPM7 channel activity is up-regulated in oxygen glucose deprived cortical neurons and that knockdown of TRPM7 expression by RNA interference in cultured neurons and the hippocampus delayed anoxic cell death. To better understand the mechanism by which the channel contributes to the demise of cells under cellular stress, we characterized the cellular effects produced by increased TRPM7 channel activity employing HEK-293 cells as a model. Overexpression of the channel-kinase in HEK-293 cells produced cell rounding that was dependent upon the calcium-dependent protease m-calpain. More recently, we have shown that TRPM7 activates m-calpain through reactive oxygen species 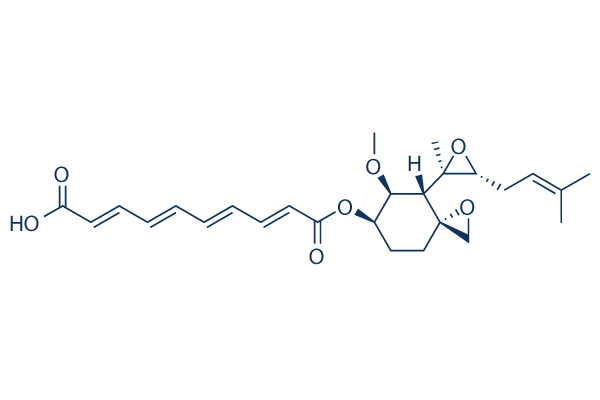 dependent activation of the stress-activated protein kinases p38 MAP kinase and c-Jun N-terminal kinase in HEK-293 cells. During the course of our investigation into the mechanism by which overexpression of TRPM7 caused loss of cell adhesion, we discovered that application of the non-specific lipoxygenase inhibitor NDGA attenuated cell rounding and loss of adhesion produced by influx of divalent cations through the channel. Electrophysiological measurements demonstrated that application of NDGA potently inhibited TRPM7 channel activity, suggesting that a lipoxygenase may be involved in regulating the channel. Lipoxygenases are a family of calciumdependent dioxygenases, including 5-lipoxygenase, 12lipoxygenase, and 15-lipoxygenase, that metabolize arachidonic acid to distinct biologically active fatty acid hydroperoxides, such as leukotrienes and hydroyeicosateraenoic acids. These metabolites take part in numerous cell processes, including inflammation, proliferation, cell invasion, angiogenesis, cell adhesion, and cell spreading. It is well known that lipid molecules modulate several members of the TRP family. In particular, products of lipoxygenase have been demonstrated to directly modulate TRPV1 channel activity. Activation of histamine and bradykinin receptors stimulates TRPV1 channel activity in a 12-LOX-dependent manner. Thus, our Trichostatin A discovery that NDGA blocked TRPM7 channel activity initially suggested to us that TRPM7 channel activity may be similarly controlled by a lipoxygenase. However, results from our experiments indicate that these compounds block TRPM7 channel activity independent of their actions on 5-LOX. Here we identify the 5-LOX inhibitors NDGA, AA861, and MK886 as potent blockers of the TRPM7 channel and demonstrate that depletion of TRPM7 channel activity by RNA interference or by treatment of cells with TRPM7 channel blockers reduces cell death caused by apoptotic stimuli. Stroke is the third leading cause of death in the United States as well as a major cause of disability. Considerable efforts have been spent on MK-0683 developing treatments for stroke, but they have been met with limited success. For example, the contribution of excitotoxicity mediated by glutamatergic NMDA receptors to ischemia-induced cell death is well appreciated, however, blockers of these receptors are not well tolerated and are only effective for a very short time following the onset of ischemia. Thus, the identification of new targets for pharmacological intervention in stroke is urgently needed. Two studies by the same group have highlighted the importance of TRPM7 to ischemic cell death.
dependent activation of the stress-activated protein kinases p38 MAP kinase and c-Jun N-terminal kinase in HEK-293 cells. During the course of our investigation into the mechanism by which overexpression of TRPM7 caused loss of cell adhesion, we discovered that application of the non-specific lipoxygenase inhibitor NDGA attenuated cell rounding and loss of adhesion produced by influx of divalent cations through the channel. Electrophysiological measurements demonstrated that application of NDGA potently inhibited TRPM7 channel activity, suggesting that a lipoxygenase may be involved in regulating the channel. Lipoxygenases are a family of calciumdependent dioxygenases, including 5-lipoxygenase, 12lipoxygenase, and 15-lipoxygenase, that metabolize arachidonic acid to distinct biologically active fatty acid hydroperoxides, such as leukotrienes and hydroyeicosateraenoic acids. These metabolites take part in numerous cell processes, including inflammation, proliferation, cell invasion, angiogenesis, cell adhesion, and cell spreading. It is well known that lipid molecules modulate several members of the TRP family. In particular, products of lipoxygenase have been demonstrated to directly modulate TRPV1 channel activity. Activation of histamine and bradykinin receptors stimulates TRPV1 channel activity in a 12-LOX-dependent manner. Thus, our Trichostatin A discovery that NDGA blocked TRPM7 channel activity initially suggested to us that TRPM7 channel activity may be similarly controlled by a lipoxygenase. However, results from our experiments indicate that these compounds block TRPM7 channel activity independent of their actions on 5-LOX. Here we identify the 5-LOX inhibitors NDGA, AA861, and MK886 as potent blockers of the TRPM7 channel and demonstrate that depletion of TRPM7 channel activity by RNA interference or by treatment of cells with TRPM7 channel blockers reduces cell death caused by apoptotic stimuli. Stroke is the third leading cause of death in the United States as well as a major cause of disability. Considerable efforts have been spent on MK-0683 developing treatments for stroke, but they have been met with limited success. For example, the contribution of excitotoxicity mediated by glutamatergic NMDA receptors to ischemia-induced cell death is well appreciated, however, blockers of these receptors are not well tolerated and are only effective for a very short time following the onset of ischemia. Thus, the identification of new targets for pharmacological intervention in stroke is urgently needed. Two studies by the same group have highlighted the importance of TRPM7 to ischemic cell death.
Increased trimethylation at H3K9 and decreased acetylation neurosensorial deafness further increase disability
A hypertrophic cardiomyopathy, present in most cases, may become symptomatic and even cause premature death. Other common problems include kyphoscoliosis, pes cavus, and, in 10 % of patients, diabetes mellitus. FRDA is caused by partial deficiency of the mitochondrial protein frataxin. Though the function of GANT61 frataxin is still partly controversial, there is general agreement that it is involved in cellular iron homeostasis and that its deficiency results in multiple enzyme deficits, mitochondrial dysfunction and oxidative damage. Frataxin binds ferrous iron through negatively charged amino acids on its surface, it promotes the mitochondrial synthesis of ironcontaining molecules, in particular iron-sulfur clusters and heme, and it controls the ability of iron to perform redox chemistry. Frataxin deficiency significantly affects ISC synthesis and results in reduced activities of several enzymes that require ISCs as prosthetic groups. Frataxin may also have a more general protective effect against oxidative stress and in determining antioxidant responses, even in the absence of excess iron. Complete absence of 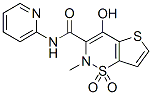 frataxin is incompatible with life in higher organisms, as demonstrated by the embryonic lethality observed in systemic gene knock-out models and by the eventual loss of cells targeted for frataxin gene deletion in conditional knock-out models. The human disease is caused by the pathological hyperexpansion of a GAA?TTC repeat sequence, ranging from 60�C1700 repeats, in the first intron of the frataxin gene that partially suppresses FXN gene expression. This WY 14643 50892-23-4 mutation is present at the homozygous state in most patients and in compound heterozygosity with a different loss-of-function mutation in a small minority of cases. In FRDA patients, frataxin amounts vary between 5% and 30% of those of normal individuals, and are little more than 50% of normal in heterozygous FRDA carriers, who have no sign of disease. These findings suggest that restoring FXN gene expression in FRDA patients to heterozygote levels may substantially slow the course of the disease. In order to develop treatments to reduce or eliminate FXN transcriptional silencing, it is necessary to understand the underlying mechanisms. In vitro and in bacterial plasmids, pathological lengths of GAA repeats adopt a non-B, triple helical DNA structure that blocks and sequesters the advancing RNA polymerase. The same repeats, when linked to a reporter gene in transgenic mice and in cells from FRDA patients, become associated with transcriptionally silent heterochromatin. Therefore, decondensing the chromatin structure at the GAA repeat expansion appears an appealing target for FRDA therapeutics. Since deacetylated histones are generally associated with silent heterochromatin, HDAC inhibitors have the potential to make heterochromatin revert to an open, active conformation that allows gene expression. The increase in FXN transcription is accompanied by increased acetylation of histone H3 at lysine14, as well as at H4K5 and H4K12 near the GAA repeat. As a further step to evaluate these drugs as potential FRDA therapeutics,we have nowinvestigated the efficacyand acutetoxicity of a compound from this novel family of HDACI in a FRDA mouse model. Our data indicate that in a mouse model that carries expanded GAA repeats in the endogenous frataxin gene, a member of this class of HDACI is able to restore frataxin levels and the gene expression profile to those of wild-type mice. In the present study we have demonstrated the in vivo feasibility of a therapeutic approach to activate the FXN gene in a mouse model that recapitulates the genetic and epigenetic features of FRDA. Previous work has shown that FXN silencing in FRDA is likely to be the consequence of chromatin changes induced by the expanded intronic GAA repeat. Post-translational modifications of histone tails are thought to form a code, called the histone code, that affect gene expression by providing binding sites for proteins involved in controlling chromatin condensation and transcription.
frataxin is incompatible with life in higher organisms, as demonstrated by the embryonic lethality observed in systemic gene knock-out models and by the eventual loss of cells targeted for frataxin gene deletion in conditional knock-out models. The human disease is caused by the pathological hyperexpansion of a GAA?TTC repeat sequence, ranging from 60�C1700 repeats, in the first intron of the frataxin gene that partially suppresses FXN gene expression. This WY 14643 50892-23-4 mutation is present at the homozygous state in most patients and in compound heterozygosity with a different loss-of-function mutation in a small minority of cases. In FRDA patients, frataxin amounts vary between 5% and 30% of those of normal individuals, and are little more than 50% of normal in heterozygous FRDA carriers, who have no sign of disease. These findings suggest that restoring FXN gene expression in FRDA patients to heterozygote levels may substantially slow the course of the disease. In order to develop treatments to reduce or eliminate FXN transcriptional silencing, it is necessary to understand the underlying mechanisms. In vitro and in bacterial plasmids, pathological lengths of GAA repeats adopt a non-B, triple helical DNA structure that blocks and sequesters the advancing RNA polymerase. The same repeats, when linked to a reporter gene in transgenic mice and in cells from FRDA patients, become associated with transcriptionally silent heterochromatin. Therefore, decondensing the chromatin structure at the GAA repeat expansion appears an appealing target for FRDA therapeutics. Since deacetylated histones are generally associated with silent heterochromatin, HDAC inhibitors have the potential to make heterochromatin revert to an open, active conformation that allows gene expression. The increase in FXN transcription is accompanied by increased acetylation of histone H3 at lysine14, as well as at H4K5 and H4K12 near the GAA repeat. As a further step to evaluate these drugs as potential FRDA therapeutics,we have nowinvestigated the efficacyand acutetoxicity of a compound from this novel family of HDACI in a FRDA mouse model. Our data indicate that in a mouse model that carries expanded GAA repeats in the endogenous frataxin gene, a member of this class of HDACI is able to restore frataxin levels and the gene expression profile to those of wild-type mice. In the present study we have demonstrated the in vivo feasibility of a therapeutic approach to activate the FXN gene in a mouse model that recapitulates the genetic and epigenetic features of FRDA. Previous work has shown that FXN silencing in FRDA is likely to be the consequence of chromatin changes induced by the expanded intronic GAA repeat. Post-translational modifications of histone tails are thought to form a code, called the histone code, that affect gene expression by providing binding sites for proteins involved in controlling chromatin condensation and transcription.
The gene is highly expressed in the oviduct under control of steroid hormones as well as in macrophages
To contribute to this effort, we have developed a high-throughput assay for PBPs that measures the fluorescence polarization of Bocillin-FL and used it to screen for potential inhibitors of N. gonorrhoeae PBP 2 from a library of 50,080 compounds. After eliminating those that acted promiscuously, did not demonstrate a concentration-dependent inhibition, or were b-lactams, 18 compounds were examined in more detail, and 7 of these exhibited antimicrobial activity against N. gonorrhoeae, including PenR and CephI strains. To the best of our knowledge, this is the first report of high-throughput screening against a PBP. Interestingly, this compound contains a thiazolidine ring, a feature of penicillin antibiotics and arylalkylidene iminothiazolidin-4-ones, which inhibit some PBPs in vitro and shows antimicrobial activity. Compound 7 was also successful in the docking simulations and there are predicted interactions with Ser310. The resemblance of compound 7 to a b-lactam-like compound led us to determine whether there is a covalent interaction with PBP 2, but no increase in mass of the protein was observed by mass spectrometry. Finally, compound 3 is probably the least promising candidate at this stage since it has a relatively high IC50 against PBP 2 and its MIC values against the N. gonorrhoeae strains are correspondingly weak. Overall, there was relatively weak correlation between IC50 and MIC values for the seven compounds, but this is expected for initial hits from HTS because there are a variety of factors that determine the MIC. Since N. gonorrhoeae expresses two essential PBPs, PBP 1 could be the lethal target for a given compound in addition to, or independent of, PBP 2. The outer membrane of Gram-negative bacteria is a SP600125 molecular weight barrier to hydrophobic compounds and the lipooligosaccharide structure of N. gonorrhoeae also impacts permeability. In addition, entry of hydrophilic compounds into the periplasmis influenced by porins and the action of efflux systems can remove compounds from the periplasm. Both of the antibiotic resistant strains contain a mutation in the promoter of mtrCDE that increases expression of the MtrC-MtrD-MtrE effluxpump. The development of this assay paves the way to screen using larger compound libraries and against variants of PBP 2 that contribute to third-generation cephalosporin resistance. They catalyze the hydrolysis of b- glycosidic bonds between the N-acetylmuramic acid and Nacetylglucosamine repeating units composing the backbone of peptidoglycan, the major constituent of bacterial cell walls. Lysozyme is a component of both phagocytic and secretory granules of neutrophils and is also produced by monocytes, macrophages and epithelial cells. It is found in significant concentrations in saliva, airway mucus, milk and other secretions, and is considered to be an important first line barrier against bacterial infection. While many gram-positive bacteria are rapidly killed by lysozyme in vitro, gram-negative bacteria are not because they have an outer membrane that prevents direct access of lysozyme to the peptidoglycan sacculus. However, in vivo, gramnegative bacteria are sensitized to lysozyme by accessory antimicrobial peptides of the  innate immunity system such as defensins and complement which disrupt the outer membrane barrier. Several structurally different lysozymes have been described and the major types within the animal kingdom are the c-type, the R428 g-type and the i-type lysozymes. Vertebrates have genes for both c- and g-type lysozyme, but their spatio-temporal expression is species-specific. The chicken genome for instance comprises a single c-type and two g-type lysozyme genes.
innate immunity system such as defensins and complement which disrupt the outer membrane barrier. Several structurally different lysozymes have been described and the major types within the animal kingdom are the c-type, the R428 g-type and the i-type lysozymes. Vertebrates have genes for both c- and g-type lysozyme, but their spatio-temporal expression is species-specific. The chicken genome for instance comprises a single c-type and two g-type lysozyme genes.