Chronic kidney disease and moreover, end-stage renal disease, have been shown to increase cardiovascular disease and risk of death. This has been substantiated in a systematic review on mortality risk, which concluded that increased risk for all-cause mortality in CKD high content screening supply patients was largely driven by cardiovascular deaths. Glucagon-like peptide-1 is an incretin hormone secreted by the small intestine in response to nutrient ingestion. Although the major physiological function of GLP-1 appears to relate to glycaemic control, evidence suggests that GLP-1 plays an important role in the cardiovascular system. GLP-1 receptors are expressed in the heart and vasculature of rodents as well as humans. Research has shown that GLP-1R agonists BIBW2992 affect a wide range of cardiovascular parameters, including heart rate, blood pressure, vascular tone and myocardial contractility. Importantly, these agents may also have beneficial effects in the setting of cardiovascular disease. For example, GLP-1 has been found to exert cardioprotective actions in experimental models of dilated cardiomyopathy, hypertensive heart disease and myocardial infarction. Preliminary clinical studies also suggest that GLP-1 infusion may improve cardiac contractile function in chronic heart failure patients with and without diabetes, and in MI patients after successful angioplasty. However, the cardiovascular effects of a pharmacological increase in GLP-1 in patients with CKD have not been determined. Dipeptidyl peptidase-4 inhibitors are considered incretin enhancers, because they inhibit the enzymatic degradation of incretins, in particular, GLP-1 and therefore are established therapies for type 2 diabetes. At the same time, DPP-4 inhibition does not cause hypoglycemia, as was previously shown by Bergman et al in a study in healthy male volunteers. Because the action of GLP-1 on insulin secretion is strictly glucose dependent, the risk of hypoglycaemia associated with DPP-4 inhibitors is low.The main elimination route of the first generation of approved DPP-4 inhibitors is via the kidney. Dose adjustment in patients with diabetes and chronic renal failure is thus necessary. Linagliptin a recently launched DPP-4 inhibitor is different in this respect with primary elimination via the bile and only 1-5% eliminated via the urine. We studied the 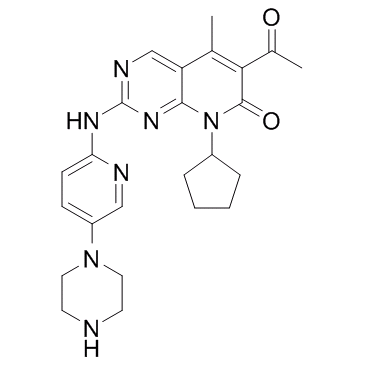 pharmacokinetics and pharmacodynamics of different DPP-4 inhibitors, in the settings of CRF, in order to determine the properties of DPP-4 inhibitors to be used in patients with impaired renal function, and investigated the effects of linagliptin on biomarkers of cardiac and renal fibrosis. The results showed that DPP-4 inhibition increases plasma GLP-1 levels, particularly in uremia, suggesting that linagliptin may offer a unique approach for treating uremic cardiomyopathy in CKD patients. The overall goal of the present study was to compare the pharmacokinetic properties of available DPP-4 inhibitors in a rat model of uremic heart disease and select the optimal compound based on these data for the first pharmacodynamics analyses of potential efficacy in this rat model. We have shown that renal impairment does not affect the pharmacokinetics of linagliptin, whereas it increases the exposure of sitagliptin and alogliptin. In the present study, only linagliptin was found not to further aggravate pathological changes of glomerular and tubular markers in rats with CRF, suggesting that it is a safe approach to be used in patients with CRF. Consequently, linagliptin was also the compound of choice to investigate further effects on uremic cardiomyopathy. This is of potential clinical impact, since patients with advanced stages of renal impairment are characterized by a high overall cardiac morbidity and mortality.
pharmacokinetics and pharmacodynamics of different DPP-4 inhibitors, in the settings of CRF, in order to determine the properties of DPP-4 inhibitors to be used in patients with impaired renal function, and investigated the effects of linagliptin on biomarkers of cardiac and renal fibrosis. The results showed that DPP-4 inhibition increases plasma GLP-1 levels, particularly in uremia, suggesting that linagliptin may offer a unique approach for treating uremic cardiomyopathy in CKD patients. The overall goal of the present study was to compare the pharmacokinetic properties of available DPP-4 inhibitors in a rat model of uremic heart disease and select the optimal compound based on these data for the first pharmacodynamics analyses of potential efficacy in this rat model. We have shown that renal impairment does not affect the pharmacokinetics of linagliptin, whereas it increases the exposure of sitagliptin and alogliptin. In the present study, only linagliptin was found not to further aggravate pathological changes of glomerular and tubular markers in rats with CRF, suggesting that it is a safe approach to be used in patients with CRF. Consequently, linagliptin was also the compound of choice to investigate further effects on uremic cardiomyopathy. This is of potential clinical impact, since patients with advanced stages of renal impairment are characterized by a high overall cardiac morbidity and mortality.
Demonstrated for the first time that short-term treatment with all DPP-4 inhibitors decreases the plasma concentrat
Leave a reply