In general, it is astonishing how few mutations were observed overall in the 228 patients of the study who have failed therapy. Only 43% of patients had any drug resistance-associated mutation detected. Missing drug pressure due to poor adherence could be a LDN-193189 supply possible explanation for the low prevalence of mutations but it is probably not the major reason because.75% of patients reported to have an excellent adherence. Nevertheless, the prevalence of resistance might be underestimated. Currently used genotypic resistance tests have a population detection limit of only,20%. Additional resistant virus variants might be present at lower levels. The late and rare occurrence of PI/r mutations can be explained by their high genetic barrier compared to NNRTIs. However, the mechanism explaining the lack of resistance to 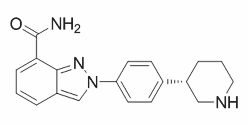 co-administered NRTIs remains unknown. It can be speculated that the two drug classes may have different activities in different anatomical compartments, with regards to free versus cell-cell virus transmissionso that the activity of PI/r might be sufficient to suppress NRTI resistant strains to undetectable levels. It could also be possible that NNRTIs, as they target the same gene as NRTIs, might select for yet unidentified compensatory mutations in the RT connection-, respectively, RNase TWS119 H-domain of the pol gene, subsequently leading to more rapid emergence of NRTI mutations. In theory, the presence of minority variants harboring NNRTI- or NRTI-drug resistant mutations, which have been detected in drug naive HIV-1 infected patients, could have a more severe impact in a regimen that contains a “low genetic barrier” drug rather than a PI/r. This aspect cannot be excluded in the present study.. Poorer adherence in the PI/r-treated group could also possibly explain the differencesbut adherence was excluded as potential bias in a sensitivity analysis. In addition, different NRTI backbones in PI/r- and NNRTI-treated individuals might have influenced our results. To disprove this concern, we performed a sensitivity analysis only including patients with a TDF/FTC backbone and we adjusted the logistic regression for the NRTI backbone. Although our study initially considered 5959 patients who started first-line cART, only 228 individuals qualified for our study. The sample size was too small to compare different treatment regimens in more detail. Unfortunately, sufficient longitudinal resistance data from our patients were not available; otherwise dynamics of evolution of individual drug resistance mutations could have been investigated in more detail. In addition, we cannot exclude that there are resistance associated mutations outside the sequenced region. No phenotypic resistance tests were available that could prove that viruses which do not harbor any mutations are really sensitive to the drugs. In conclusion, PI/r containing cART leads to long-lasting protection of the activity of NRTIs and PI/r despite ongoing viral replication after virological failure. Accumulation of drug resistance mutations against all three drugs of the regimen is slower and less frequent when compared to NNRTI-containing regimens, thus retaining more options for second-line therapy. These findings are of high relevance for settings, which lack the opportunities for regular virological monitoring and where the use of PI/r as first-line therapies should be considered. Inhibiting proteasome function has been demonstrated as a novel therapeutic strategy in multiple disease models like fibrosis, inflammation, ischemia-reperfusion injury and cancer. Proteasome inhibitor bortezomibhas been approved by the United States Food and Drug Administration to treat multiple myeloma.
co-administered NRTIs remains unknown. It can be speculated that the two drug classes may have different activities in different anatomical compartments, with regards to free versus cell-cell virus transmissionso that the activity of PI/r might be sufficient to suppress NRTI resistant strains to undetectable levels. It could also be possible that NNRTIs, as they target the same gene as NRTIs, might select for yet unidentified compensatory mutations in the RT connection-, respectively, RNase TWS119 H-domain of the pol gene, subsequently leading to more rapid emergence of NRTI mutations. In theory, the presence of minority variants harboring NNRTI- or NRTI-drug resistant mutations, which have been detected in drug naive HIV-1 infected patients, could have a more severe impact in a regimen that contains a “low genetic barrier” drug rather than a PI/r. This aspect cannot be excluded in the present study.. Poorer adherence in the PI/r-treated group could also possibly explain the differencesbut adherence was excluded as potential bias in a sensitivity analysis. In addition, different NRTI backbones in PI/r- and NNRTI-treated individuals might have influenced our results. To disprove this concern, we performed a sensitivity analysis only including patients with a TDF/FTC backbone and we adjusted the logistic regression for the NRTI backbone. Although our study initially considered 5959 patients who started first-line cART, only 228 individuals qualified for our study. The sample size was too small to compare different treatment regimens in more detail. Unfortunately, sufficient longitudinal resistance data from our patients were not available; otherwise dynamics of evolution of individual drug resistance mutations could have been investigated in more detail. In addition, we cannot exclude that there are resistance associated mutations outside the sequenced region. No phenotypic resistance tests were available that could prove that viruses which do not harbor any mutations are really sensitive to the drugs. In conclusion, PI/r containing cART leads to long-lasting protection of the activity of NRTIs and PI/r despite ongoing viral replication after virological failure. Accumulation of drug resistance mutations against all three drugs of the regimen is slower and less frequent when compared to NNRTI-containing regimens, thus retaining more options for second-line therapy. These findings are of high relevance for settings, which lack the opportunities for regular virological monitoring and where the use of PI/r as first-line therapies should be considered. Inhibiting proteasome function has been demonstrated as a novel therapeutic strategy in multiple disease models like fibrosis, inflammation, ischemia-reperfusion injury and cancer. Proteasome inhibitor bortezomibhas been approved by the United States Food and Drug Administration to treat multiple myeloma.
Monthly Archives: July 2019
AChE are highly structurally constrainedand the G119S mutation is widely distributed worldwide in mosquitoes
This hypothesis should be fully tested in future studies in a number of cell lines and  in vivo model systems to conclusively determine the mechanism of sensitivity. As to whether NU9056 is generally toxic to the cells; we believe that this is unlikely for the most part. Firstly, there was no change in cH2AX staining when NU9056 was applied to cells suggesting no induction of DNA damage. Secondly differential effects were seen depending on the cell line suggesting generally toxicity was not the cause of detrimental cellular effects. However, at higher doses a role for general toxicity may become apparent. Overall, a therapeutic role for Tip60 inhibitors in the treatment of castrate resistant CaP is supported by the chemical biology and molecular genetic studies described in this paper. These molecules act on the nervous system through inhibition of acetylcholinesteraseor voltage-gated sodium channels. The major setback of insecticide use is the selection for resistance, observed not only in the targeted pests but also in many other sympatric species. At the physiological level, resistance is a consequence of either increased detoxication or modification of the insecticide target, the latter often resulting in very high insensitivity. However both mechanisms may be responsible for vector control failure and have to be addressed by insecticide resistance management strategies. Resistance has spread to such an extent, particularly in mosquito vector populations, that it now represents a critical issue for the control of the diseases they transmit, e.g. malaria, dengue, filariasis, West Nile fever or Japanese encephalitis. Sustainable strategies to counter resistance spread aim at maintaining resistant alleles at frequencies low enough so that current insecticides remain efficient even at moderate doses. As an example, the reasoned use of insecticides through rotations or mosaic applications takes advantage of the pleiotropic costto maintain resistant alleles at low frequencies. Essentially used for malaria control, fungi also represent promising tools because they kill mosquitoes at slower rate than insecticides thus reducing the risk of resistance selection. Here we propose an alternative approach based on the development of “resistant killer” compounds, capable of preferentially inhibiting targets already insensitive to a given insecticide class. Combined with the fitness cost already associated with resistance, populations treated with such “resistant killers” are thus expected to regain a high frequency of susceptible wild type alleles, a “hit where it already hurts” strategy. Ideally, the targeted protein should be highly SAR131675 constrained structurally to minimize its capacity to evolve through the selection of new mutations that would Ruxolitinib 941678-49-5 confer resistance to both the insecticide and the “resistant killer” compound. A good candidate is acetylcholinesterase, which in Coelomates acts as a synaptic terminator of nerve impulses through hydrolysis of the neurotransmitter acetylcholine. Mosquitoes contain two AChE genes, ace-1 encoding the synaptic enzyme. So far, only three substitutions on residues lining the catalytic site confer OP and CX insensitivity to AChE1: the F331W substitution, found only in Culex tritaeniorhynchus, the F290V substitution, found only in C. pipiens species, and the universally found G119S substitution, which confers the highest level of insensitivity to a broad range of insecticides and was selected independently in several Culex and Anopheles species. The G119S-substituted AChE1 appeared as a suitable candidate for the development of reverser compounds because associated with a substantial.
in vivo model systems to conclusively determine the mechanism of sensitivity. As to whether NU9056 is generally toxic to the cells; we believe that this is unlikely for the most part. Firstly, there was no change in cH2AX staining when NU9056 was applied to cells suggesting no induction of DNA damage. Secondly differential effects were seen depending on the cell line suggesting generally toxicity was not the cause of detrimental cellular effects. However, at higher doses a role for general toxicity may become apparent. Overall, a therapeutic role for Tip60 inhibitors in the treatment of castrate resistant CaP is supported by the chemical biology and molecular genetic studies described in this paper. These molecules act on the nervous system through inhibition of acetylcholinesteraseor voltage-gated sodium channels. The major setback of insecticide use is the selection for resistance, observed not only in the targeted pests but also in many other sympatric species. At the physiological level, resistance is a consequence of either increased detoxication or modification of the insecticide target, the latter often resulting in very high insensitivity. However both mechanisms may be responsible for vector control failure and have to be addressed by insecticide resistance management strategies. Resistance has spread to such an extent, particularly in mosquito vector populations, that it now represents a critical issue for the control of the diseases they transmit, e.g. malaria, dengue, filariasis, West Nile fever or Japanese encephalitis. Sustainable strategies to counter resistance spread aim at maintaining resistant alleles at frequencies low enough so that current insecticides remain efficient even at moderate doses. As an example, the reasoned use of insecticides through rotations or mosaic applications takes advantage of the pleiotropic costto maintain resistant alleles at low frequencies. Essentially used for malaria control, fungi also represent promising tools because they kill mosquitoes at slower rate than insecticides thus reducing the risk of resistance selection. Here we propose an alternative approach based on the development of “resistant killer” compounds, capable of preferentially inhibiting targets already insensitive to a given insecticide class. Combined with the fitness cost already associated with resistance, populations treated with such “resistant killers” are thus expected to regain a high frequency of susceptible wild type alleles, a “hit where it already hurts” strategy. Ideally, the targeted protein should be highly SAR131675 constrained structurally to minimize its capacity to evolve through the selection of new mutations that would Ruxolitinib 941678-49-5 confer resistance to both the insecticide and the “resistant killer” compound. A good candidate is acetylcholinesterase, which in Coelomates acts as a synaptic terminator of nerve impulses through hydrolysis of the neurotransmitter acetylcholine. Mosquitoes contain two AChE genes, ace-1 encoding the synaptic enzyme. So far, only three substitutions on residues lining the catalytic site confer OP and CX insensitivity to AChE1: the F331W substitution, found only in Culex tritaeniorhynchus, the F290V substitution, found only in C. pipiens species, and the universally found G119S substitution, which confers the highest level of insensitivity to a broad range of insecticides and was selected independently in several Culex and Anopheles species. The G119S-substituted AChE1 appeared as a suitable candidate for the development of reverser compounds because associated with a substantial.
RTK inhibitors were tested for growth suppression of their respective IC50 concentrations
Thirty-two such combinations were evaluated and VE-821 in vivo showed that single agents, did not substantially alter growth, and only certain combinations suppressed growth. The extent of growth fold inhibition was calculated by dividing the alamarBlue fluorescence values for the PF-04217903 treated cells with fluorescence values for cells treated with the vehicle. With the aim of translating the drug combinations to possible human use, we eventually focused only on drugs currently approved by the FDA that targeted the RTKs mutated in GBM. Erlotinib did not show significant inhibition even at a concentration of 100 mM. Lack of erlotinib activity may be attributed to its low solubility in DMSO compared to gefitinib and was therefore eliminated from the subsequent analysis. Remaining were these four inhibitors: gefitinib, imatinib, sunitinib and sorafenib. To test for synergistic cytotoxic effect on GBM oncospheres, one tenthand one fourth the IC50 values were next used. Single drugs and pair-wise combinations of these drugs were analyzed in GBM oncosphere lines for proliferation and caspase induction. Drug combinations containing sunitinib were best at inducing apoptosis, and the best combination for inhibiting growth appeared to be gefitinib plus sunitinib. To investigate the differences observed in the growth inhibition and caspase assay of the GBM oncospheres, the ability of the oncospheres to recover and proliferate following treatment with RTK inhibitors was analyzed. Cells were treated with RTK inhibitors at 25% of IC50 concentrations for 24 hours. The drugs were withdrawn after 24 hours and the ability of the oncospheres to regrow was assessed after two additional weeks of culture in the growth media using alamar blue cell growth assay. The growth assessment revealed that oncospheres treated with single agents or with the combination of RTK inhibitors were able to regrow with the exception of the cells treated with the combination of gefitinib and sunitinib. Moreover, observation of the cells treated with the drugs with light microscopy revealed that cells treated either with single agents or combinations of RTK inhibitors other than gefitinib and sunitinib were able to form oncospheres, whereas the cells treated with a combination of gefitinib and sunitinib were unable to form oncospheres. 020913 cells formed fewer neurospheres when treated with sunitinib compared to gefitinib reflecting the growth inhibition seen earlier in alamar blue assay. This observation suggests that gefitinib and sunitinib forms a specific combination that effectively inhibits growth of GBM oncospheres. The goal of this work was to determine if we could find a combination of approved RTK inhibitors that might be superior to single agent therapy, and test this combination in preclinical animal models of glioblastoma. Monotherapy of RTK targeting agents have been largely ineffective and there is enough in vitro experimental evidence to support the use of combination therapy targeting multiple tyrosine kinases. We first identified possible effective RTK combination using in vitro cell proliferation studies. Next we planned to test efficacy in improved preclinical animal models at FDA approved doses to try and mimic what might be achievable in a clinical trial. In this study, gefitinib and sunitinib was the best in vitro combination, based on its ability to reduce proliferation 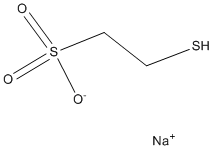 and kill GBM oncospheres. The pattern of effective inhibitor combinations suggests that successful simultaneous inhibition of EGFR and PDGFR and other tyrosine kinases was necessary.
and kill GBM oncospheres. The pattern of effective inhibitor combinations suggests that successful simultaneous inhibition of EGFR and PDGFR and other tyrosine kinases was necessary.
Control of ubiquitination also plays an established role in targeting for the altered levels of intracellular peptides observed
The therapeutic effect of bortezomib and carfilzomib as anticancer drugs is generally considered to be through alteration of protein turnover. However, these drugs produce a rapid and dramatic change in the cellular peptidome, increasing the levels of some peptides and decreasing the levels of other peptides. If these peptides are biologically active, the changes in peptide levels could contribute to the physiological effects of the drugs. Several studies have shown that intracellular peptides can influence signal transduction pathways. Many other studies have shown that synthetic peptides of 10�C20 amino acids can perturb a number of processes within the cell. Therefore, it is possible that the therapeutic and/or side effects of bortezomib and carfilzomib are mediated in part through the changes in the cellular peptidome. Resistance to LY2157299 antibiotics has become increasingly common among bacterial pathogens over the past few decades. For example, our resources to treat infections with extensively drugresistant Mycobacterium tuberculosis are extremely limited and require a therapy based on a combination of different classes of antibiotics. The emerging class of antibiotic-resistant bacteria, the carbapenem-resistant Enterobacteriaceae, which kills almost half of infected patients, is also a major health concern as all antibiotics currently available are ineffective. Despite this trend, the antibacterial drug development pipeline flow is low and the number of new drugs available is rapidly decreasing. With notable increases in antibiotic resistance, the aging of the population and the fact that infectious diseases remain one of the leading causes of death worldwide, there is an urgent need for additional and diverse therapeutic strategies to treat infections. Promising approaches for treatment of infectious diseases have been emerging. These include anti-virulence agents that target bacterial virulence determinants, or host-directed therapies, such as immunomodulatory drugs that enhance host immunity to promote more effective anti-microbial attack. Hosttargeted approaches possess major advantages compared to classic antibiotics that aim to kill or reduce bacterial growth, such as reducing selection for resistance genotypes, as there is less or no selective pressure directly imposed on the pathogen. TWS119 Moreover, stimulation of the innate immune response may provide broadspectrum protection against a range of pathogenic microorganisms, including bacteria, virus and parasites. Host-directed therapies may be used as adjunct treatments to synergize with commonly used anti-microbial drugs and may also allow diversification of therapeutic strategies currently available. Protein ubiquitination is a reversible post-translational modification that regulates diverse cellular processes, 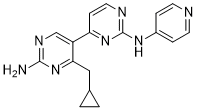 such as DNA repair, cell division, signaling, protein degradation and notably, innate immune function. Ubiquitination occurs by covalent attachment of an,8.5 kDa ubiquitin molecule to a lysine residue in the target protein by the sequential action of three enzymes; a ubiquitin-activating enzyme, a ubiquitin-conjugating enzyme and a ubiquitin-ligase enzyme. Ubiquitin is removed from proteins by deubiquitinases by proteolysis. The human genome encodes over 100 proteins that possess putative DUB activity but physiological substrates of these proteins remain poorly defined for most. DUB enzymes have established roles in a broad spectrum of diseases such as cancer, viral infection and neurodegenerative disorders. Although the function of most DUBs in immune regulation is not known, a few are key players in the modulation of innate immunity and inflammation. For example, the deubiquitinases, A20 and CYLD, control NF-kB signaling, a critical pathway in immunity and cell survival.
such as DNA repair, cell division, signaling, protein degradation and notably, innate immune function. Ubiquitination occurs by covalent attachment of an,8.5 kDa ubiquitin molecule to a lysine residue in the target protein by the sequential action of three enzymes; a ubiquitin-activating enzyme, a ubiquitin-conjugating enzyme and a ubiquitin-ligase enzyme. Ubiquitin is removed from proteins by deubiquitinases by proteolysis. The human genome encodes over 100 proteins that possess putative DUB activity but physiological substrates of these proteins remain poorly defined for most. DUB enzymes have established roles in a broad spectrum of diseases such as cancer, viral infection and neurodegenerative disorders. Although the function of most DUBs in immune regulation is not known, a few are key players in the modulation of innate immunity and inflammation. For example, the deubiquitinases, A20 and CYLD, control NF-kB signaling, a critical pathway in immunity and cell survival.
The majority of advanced pancreatic tumors harbor short telomeres and chromosomal after conventional cancer therapy
Not only do they selectively target the telomerasepositive Vismodegib cancer cells, but their growth inhibitory effects increase as the targeted cells perform an increasing number of cell divisions. In the present study, we have characterized the effects of a telomerase inhibitor, GRN163L, on the cellular lifespan and survival of a panel of pancreatic cancer cell lines. Telomerase is the enzyme responsible for the maintenance of telomeres, essential structures that cap and protect the ends of linear chromosomes. Human telomeres are made of tandem copies of n DNA repeats and of associated proteins, which together form a protective capping complex. This cap protects chromosomal ends from degradation, interchromosomal fusions and from being recognized as double-stranded DNA breaks, a form of DNA damage. Because of problems associated with the replication of the ends of linear DNA molecules, the so-called end-replication 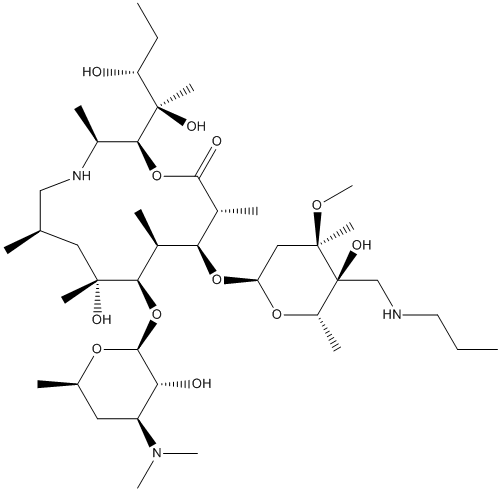 problems, telomeres shorten each time human somatic cells divide and this attrition limits their lifespan. Once the shortest telomere become uncapped, a DNA damage response is induced that mobilizes the p53 and p16/pRB pathways, which then act together to induce senescence, a viable state of irreversible quiescence. If the p53 and p16/pRB pathways are disabled, the cells will ignore these growth inhibitory signals and will continue to divide and shorten their telomeres. Eventually, terminal telomere shortening lead to crisis, a non-viable state associated with programmed cell death. Crisis is triggered by recurrent cycles of telomeretelomere fusions, anaphase bridges and chromosome breakage. When present, telomerase can prevent the induction of senescence and crisis and extend cellular lifespan by the synthesis and addition of new telomeric repeats to the telomeres. Telomerase is ubiquitously present in the early stages of human development. But by the time of birth, expression of the enzyme is repressed and telomerase becomes absent from most somatic tissues, including the pancreas. Cancer specimens, in stark contrast to normal tissues, are LEE011 almost always positive for telomerase activity, including pancreatic ductal adenocarcinomas. Detected in more than 85% of cancers, irrespective of the tumor type, telomerase is one of the best known markers of cancer cells. Moreover, this expression of telomerase in cancer cells is required for their unlimited proliferation or immortality, a hallmark of cancer. Accordingly, the inhibition of telomerase in cancer cells leads to telomere attrition and limits the lifespan of these cells. After sufficient telomere attrition has taken place, telomerase-inhibited cancer cells will succumb to either senescence or apoptosis, depending on the cellular system. This reliance on telomerase from their unlimited growth and the almost universal expression of telomerase in cancer cells make telomerase an attractive target for cancer therapy. A potential drawback, however, are the delays needed before the targeted cancer cells have lost sufficient telomeres for senescence or crisis to be induced. This delayed action might preclude their use as a first line of treatment for cancer, but to block the regrowth of residual disease after conventional therapy, telomerase inhibitors have been expected to have good therapeutic potential. Telomere shortening is the earliest and most common genetic alteration acquired during pancreatic cancer development. This alteration, detected in 96% of PanIN precursor lesions, is accompanied by evidence of a DNA damage response consistent with telomere uncapping and dysfunction. Not surprisingly, more than 90% of these tumors eventually re-activate telomerase, which otherwise remains undetectable in normal pancreatic tissues.
problems, telomeres shorten each time human somatic cells divide and this attrition limits their lifespan. Once the shortest telomere become uncapped, a DNA damage response is induced that mobilizes the p53 and p16/pRB pathways, which then act together to induce senescence, a viable state of irreversible quiescence. If the p53 and p16/pRB pathways are disabled, the cells will ignore these growth inhibitory signals and will continue to divide and shorten their telomeres. Eventually, terminal telomere shortening lead to crisis, a non-viable state associated with programmed cell death. Crisis is triggered by recurrent cycles of telomeretelomere fusions, anaphase bridges and chromosome breakage. When present, telomerase can prevent the induction of senescence and crisis and extend cellular lifespan by the synthesis and addition of new telomeric repeats to the telomeres. Telomerase is ubiquitously present in the early stages of human development. But by the time of birth, expression of the enzyme is repressed and telomerase becomes absent from most somatic tissues, including the pancreas. Cancer specimens, in stark contrast to normal tissues, are LEE011 almost always positive for telomerase activity, including pancreatic ductal adenocarcinomas. Detected in more than 85% of cancers, irrespective of the tumor type, telomerase is one of the best known markers of cancer cells. Moreover, this expression of telomerase in cancer cells is required for their unlimited proliferation or immortality, a hallmark of cancer. Accordingly, the inhibition of telomerase in cancer cells leads to telomere attrition and limits the lifespan of these cells. After sufficient telomere attrition has taken place, telomerase-inhibited cancer cells will succumb to either senescence or apoptosis, depending on the cellular system. This reliance on telomerase from their unlimited growth and the almost universal expression of telomerase in cancer cells make telomerase an attractive target for cancer therapy. A potential drawback, however, are the delays needed before the targeted cancer cells have lost sufficient telomeres for senescence or crisis to be induced. This delayed action might preclude their use as a first line of treatment for cancer, but to block the regrowth of residual disease after conventional therapy, telomerase inhibitors have been expected to have good therapeutic potential. Telomere shortening is the earliest and most common genetic alteration acquired during pancreatic cancer development. This alteration, detected in 96% of PanIN precursor lesions, is accompanied by evidence of a DNA damage response consistent with telomere uncapping and dysfunction. Not surprisingly, more than 90% of these tumors eventually re-activate telomerase, which otherwise remains undetectable in normal pancreatic tissues.