The above two Mechlorethamine hydrochloride methods are predictive approaches that provide with novel, testable small molecule-target associations, while the text mining-based approach is a way to gather information previously existing in the literature that would probably have been missed. Additionally, it also suffers from an inability to detect new 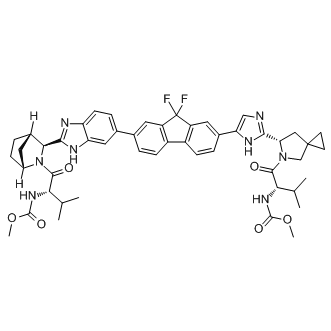 biological findings, and their efficiency is generally hampered by the redundancy of the compound and gene names in literature. Therefore, the genome-wide application of LBVS, SBVS and texting miningbased methods still has many limitations. An effective means that might overcome these problems is not to considerate each drug or target independently from other drugs or targets, but to take the standpoint of chemical genomics which could open up new opportunities to identify new drug leads or therapeutic targets instead. Chemical genomics aims at exploiting the whole chemical space, which corresponds to not only the space of the small molecules but also of those proteins interacting with the molecules. Recently, several chemical genomics approaches, including the ligand-based, targetbased or target-ligand methods have been developed to predict the interactions between compounds and proteins. The ligand-based method that integrated the protein targets was designed at the level of families or subfamilies which is appropriate for some specific protein families such as GPCRs. Based on the ligand binding site similarity, Frimurer et al developed a target-based approach which clustered the Albaspidin-AA receptors and pooled together the known ligands for each cluster to infer shared ligands. Different from these two approaches, the target-ligand approach combines the ligand chemical space, target space and the currently known drug-target networks information to construct a complex forecast system, with purpose to predict ligands or targets for a given target or ligand without prior attempting to define a special set of similar receptors or ligands. For instance, the amino acid sequences, 2-dimensional chemical structures and mass-spectrometry data have been collected together to build a statistical method for predicting compound-protein interactions based on 519 approved drugs and their 291 associated targets. Similarly, the chemical functional groups and biological features have also been adopted to establish the classification models for predicting the drug-target interaction network. Interestingly, without the negative samples, the semi- supervised machine learning algorithm NetLapRLS has been developed based on heterogeneous biological data, which could effectively predict the interaction of each chemical-protein pair. Furthermore, based on DrugBank database, the sets of chemical substructures and protein domains have also been collected and effectively analyzed using Sparse Canonical Correspondence Analysis and Support Vector Machine methods to identify molecular recognition rules between drugs and targets. However, all these aforementioned methods might suffer from the small receptor space which only focused on certain protein families or the limited chemical space only covered by the FDA approved drugs. To predict the drug-target interactions, we have designed a set of in silico tools by incorporating the chemical, genomic and pharmacological information into an integrated framework using the largest available dataset of DrugBank database. The predictions are based on extraction of conserved patterns from subdivided interaction vectors involving both proteins and their corresponding ligands.
biological findings, and their efficiency is generally hampered by the redundancy of the compound and gene names in literature. Therefore, the genome-wide application of LBVS, SBVS and texting miningbased methods still has many limitations. An effective means that might overcome these problems is not to considerate each drug or target independently from other drugs or targets, but to take the standpoint of chemical genomics which could open up new opportunities to identify new drug leads or therapeutic targets instead. Chemical genomics aims at exploiting the whole chemical space, which corresponds to not only the space of the small molecules but also of those proteins interacting with the molecules. Recently, several chemical genomics approaches, including the ligand-based, targetbased or target-ligand methods have been developed to predict the interactions between compounds and proteins. The ligand-based method that integrated the protein targets was designed at the level of families or subfamilies which is appropriate for some specific protein families such as GPCRs. Based on the ligand binding site similarity, Frimurer et al developed a target-based approach which clustered the Albaspidin-AA receptors and pooled together the known ligands for each cluster to infer shared ligands. Different from these two approaches, the target-ligand approach combines the ligand chemical space, target space and the currently known drug-target networks information to construct a complex forecast system, with purpose to predict ligands or targets for a given target or ligand without prior attempting to define a special set of similar receptors or ligands. For instance, the amino acid sequences, 2-dimensional chemical structures and mass-spectrometry data have been collected together to build a statistical method for predicting compound-protein interactions based on 519 approved drugs and their 291 associated targets. Similarly, the chemical functional groups and biological features have also been adopted to establish the classification models for predicting the drug-target interaction network. Interestingly, without the negative samples, the semi- supervised machine learning algorithm NetLapRLS has been developed based on heterogeneous biological data, which could effectively predict the interaction of each chemical-protein pair. Furthermore, based on DrugBank database, the sets of chemical substructures and protein domains have also been collected and effectively analyzed using Sparse Canonical Correspondence Analysis and Support Vector Machine methods to identify molecular recognition rules between drugs and targets. However, all these aforementioned methods might suffer from the small receptor space which only focused on certain protein families or the limited chemical space only covered by the FDA approved drugs. To predict the drug-target interactions, we have designed a set of in silico tools by incorporating the chemical, genomic and pharmacological information into an integrated framework using the largest available dataset of DrugBank database. The predictions are based on extraction of conserved patterns from subdivided interaction vectors involving both proteins and their corresponding ligands.
Monthly Archives: June 2019
The AD11 model displays progressive memory deficits and neurodegeneration as a consequence of NGF deprivation
Procedures and criteria for the Retest day were identical to those on the initial Response Discrimination day. On the following day, mice were then trained to always enter the arm that contained the visual cue, the location of which was again pseudorandomly varied in the left and right arms. Training was continued until a mouse made a correct choice on the probe trial. For each day, we analyzed the total number of trials to criterion and the number of probe trials required to reach criterion. For the Shift to Visual-Cue Learning day, errors were scored as entries into arms that did not contain the visual cue, and they were further broken down into three subcategories to determine whether CIE altered the ability to 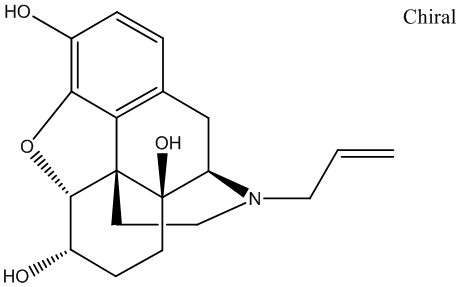 either shift from the previously learned strategy or to maintain the new strategy after perseveration had ceased. In order to detect shifts in the strategies that animals used, trials were separated into consecutive blocks of four trials each. A perseverative error occurred when a mouse made the same egocentric response as required during the Response Discrimination day, but which was opposite to the direction of the arm containing the visual cue. Six of every 12 consecutive trials required the mouse to respond in this manner. A perseverative error was scored when the mouse entered the incorrect arm on three or more trials per block of 4 trials. Once the mouse made less than three perseverative errors in a block, all subsequent errors were now scored as regressive errors. The third type of error, termed “never reinforced” errors, was scored when a mouse entered the incorrect arm on trials where the visual cue was placed on the same side that the mouse had been trained to enter on the previous day. Current strategies for NGF therapy in AD use highly invasive approaches, such as a neurosurgical intracerebroventricular injection of NGF or a parenchimal injection of cells secreting hNGF or of viruses harboring hNGF gene. To fully exploit the therapeutic potential of NGF in a noninvasive manner, its therapeutic window must be improved, by increasing the brain distribution, while limiting NGF paininducing actions. The intranasal delivery represents a viable option to non invasively increase NGF biodistribution in the brain, where it exerts anti-neurodegenerative actions. NGF intranasal delivery minimizes the build-up of peripheral NGF concentration, even if residual leakage and absorption of NGF into the blood stream, from the nasal compartment, has been shown. The fact that the NGF mutation R100W appears to separate the effects of NGF on CNS development from those involved in the activation of peripheral pain pathways, provides a basis for designing “painless” NGF variant molecules. In Benzoylaconine particular, we demonstrated that the hNGFR100E mutant displays a full neurotrophic activity in cultured neurons, while showing a reduced nociceptive activity in vivo, via a selective alteration of TrkA versus p75NTR binding and signaling. For this reason, we used the R100E hNGF mutants for in vivo studies, demonstrating that hNGFR100E has a nociceptive activity which is much weaker than that of wild type hNGF. For the present study, the R100E mutation was inserted in the context of a recombinant form of human NGF “tagged” with a single residue epitope, which replaces the Pro residue at position 61 of hNGF with Ser residue present in mouse NGF. hNGFP61S “tagged” molecules are equally Ginsenoside-Ro bioactive as hNGF and are selectively detectable against wild type hNGF, with a specific monoclonal antibody.
either shift from the previously learned strategy or to maintain the new strategy after perseveration had ceased. In order to detect shifts in the strategies that animals used, trials were separated into consecutive blocks of four trials each. A perseverative error occurred when a mouse made the same egocentric response as required during the Response Discrimination day, but which was opposite to the direction of the arm containing the visual cue. Six of every 12 consecutive trials required the mouse to respond in this manner. A perseverative error was scored when the mouse entered the incorrect arm on three or more trials per block of 4 trials. Once the mouse made less than three perseverative errors in a block, all subsequent errors were now scored as regressive errors. The third type of error, termed “never reinforced” errors, was scored when a mouse entered the incorrect arm on trials where the visual cue was placed on the same side that the mouse had been trained to enter on the previous day. Current strategies for NGF therapy in AD use highly invasive approaches, such as a neurosurgical intracerebroventricular injection of NGF or a parenchimal injection of cells secreting hNGF or of viruses harboring hNGF gene. To fully exploit the therapeutic potential of NGF in a noninvasive manner, its therapeutic window must be improved, by increasing the brain distribution, while limiting NGF paininducing actions. The intranasal delivery represents a viable option to non invasively increase NGF biodistribution in the brain, where it exerts anti-neurodegenerative actions. NGF intranasal delivery minimizes the build-up of peripheral NGF concentration, even if residual leakage and absorption of NGF into the blood stream, from the nasal compartment, has been shown. The fact that the NGF mutation R100W appears to separate the effects of NGF on CNS development from those involved in the activation of peripheral pain pathways, provides a basis for designing “painless” NGF variant molecules. In Benzoylaconine particular, we demonstrated that the hNGFR100E mutant displays a full neurotrophic activity in cultured neurons, while showing a reduced nociceptive activity in vivo, via a selective alteration of TrkA versus p75NTR binding and signaling. For this reason, we used the R100E hNGF mutants for in vivo studies, demonstrating that hNGFR100E has a nociceptive activity which is much weaker than that of wild type hNGF. For the present study, the R100E mutation was inserted in the context of a recombinant form of human NGF “tagged” with a single residue epitope, which replaces the Pro residue at position 61 of hNGF with Ser residue present in mouse NGF. hNGFP61S “tagged” molecules are equally Ginsenoside-Ro bioactive as hNGF and are selectively detectable against wild type hNGF, with a specific monoclonal antibody.
This is based on the fact that except translocation-deregulated adhered to the RACK1 RNAi cells to similar extent as to the control cells
This implies that in the absence of RACK1, eukaryotic cells are protected from the Yersinia antiphagocytic attack. Notably, the absence of RACK1 could not protect RACK RNAi cells from 3,4,5-Trimethoxyphenylacetic acid antiphagocytosis induced by the yopK null mutant or the yopKAA mutant. In contrast, RACK1 RNAi cells were resistant towards the antiphagocytic activity of a trans-complemented yopK null mutant. Pathogenic Yersinia species utilize a powerful T3SS to deliver effector proteins into the interior of the targeted host cells. A set of translocator proteins form a pore in the host cell membrane through which these effectors are thought to gain access into the host cell cytosol. This virulence mechanism allows these bacteria to purposefully block innate immune cell defense mechanisms such as phagocytosis so they can proliferate in lymphatic tissue. However, the phagocytic process is initiated as soon as a bacterium comes in contact with a target cell and completed within minutes after cell contact. Therefore, it is assumed that pathogenic Yersinia battle to impair this process via a very rapid and precise mechanism for effector delivery and action. In line with this, we have previously shown that Yop effector deployment and intracellular activity can be measured within 30 seconds after the bacterium makes contact with a host cell. Nevertheless, extreme rapidity alone would not be sufficient; individual Yops would also need targeting to the precise site of action. Regulation of this process in the bacterium occurs on several levels; this includes coordinating target cell contact with induction of gene expression, subsequent feed-back repression, and via control of effector translocation. We have presented data in this study to support a mechanism that guarantees productive antiphagocytic effector translocation leading to an instant blockage of phagocytosis. It relies upon an interaction in the host cell between YopK, which is associated with the pore complex, and the eukaryotic protein RACK1. We observed that RACK1 was specifically required for antiphagocytosis. This was based on the finding that host cells downregulated for RACK1 expression engulfed the Folinic acid calcium salt pentahydrate pathogen to a much greater extent compared to cells with normal RACK1 expression, implying that these RACK1 RNAi cells are resistant to the antiphagocytic activity of the T3SS. This was quite unexpected; making it the first reported cell line to be 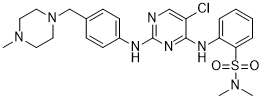 impervious to the action of the Yersinia antiphagocytic machinery. Importantly, virulence effectors were translocated to the same extent into cells regardless of their RACK1 expression level and these intracellular effectors still induced a normal cytotoxic response in both cell lines. This means two things; firstly, translocation of effectors and the process of cytotoxicity induction do not require RACK1, and secondly, antiphagocytosis and cytotoxicity are clearly two distinct events during cell infection. Another striking feature was that the ability of the pathogen to mediate antiphagocytosis towards RACK1 RNAi cells diminished significantly when YopK was present. Notably, this failure to inactivate the critical cellular targets and interrupt phagocytosis occurred despite efficient antiphagocytic Yop effector translocation into the target cells. Hence, the discrepancy between Yersinia YopK+ bacteria inducing an uncompromising cytotoxic response on the one hand, but failing to establish antiphagocytosis on the other, suggests that the latter process requires specific features of the effector translocation process that are overseen by YopK. This agrees with our previous results concerning the antiphagocytic effector and major cytotoxin YopE; certain yopE point mutants exhibited attenuated virulence but still gave rise to cytotoxicity in HeLa cells. Thus, the cytotoxic effect is actually a secondary event that occurs only after prolonged infection and is dispensable for in vivo virulence. On the other hand, there appears to be a direct correlation between the ability of Y. pseudotuberculosis to resist phagocytosis and to cause systemic infections in mice.
impervious to the action of the Yersinia antiphagocytic machinery. Importantly, virulence effectors were translocated to the same extent into cells regardless of their RACK1 expression level and these intracellular effectors still induced a normal cytotoxic response in both cell lines. This means two things; firstly, translocation of effectors and the process of cytotoxicity induction do not require RACK1, and secondly, antiphagocytosis and cytotoxicity are clearly two distinct events during cell infection. Another striking feature was that the ability of the pathogen to mediate antiphagocytosis towards RACK1 RNAi cells diminished significantly when YopK was present. Notably, this failure to inactivate the critical cellular targets and interrupt phagocytosis occurred despite efficient antiphagocytic Yop effector translocation into the target cells. Hence, the discrepancy between Yersinia YopK+ bacteria inducing an uncompromising cytotoxic response on the one hand, but failing to establish antiphagocytosis on the other, suggests that the latter process requires specific features of the effector translocation process that are overseen by YopK. This agrees with our previous results concerning the antiphagocytic effector and major cytotoxin YopE; certain yopE point mutants exhibited attenuated virulence but still gave rise to cytotoxicity in HeLa cells. Thus, the cytotoxic effect is actually a secondary event that occurs only after prolonged infection and is dispensable for in vivo virulence. On the other hand, there appears to be a direct correlation between the ability of Y. pseudotuberculosis to resist phagocytosis and to cause systemic infections in mice.
Developed myopathy and cataracts similar individuals carrying the aB-crystallin R120G mutation
The lens cataract and myopathic muscle in Catharanthine sulfate mutant mice and humans with the aB-crystallin R120G mutation shared common pathological features and molecular mechanisms. We observed significant a-crystallin aggregation in the lenses of mutant mice, which increased with cataract severity. We also found that the molecular weight of b- and c-crystallin fractions from the mutant lenses was higher than the wild-type lenses because these peaks have more light scattering, implying that b- and c-crystallin fractions aggregate more in heterozygous and homozygous mutant lenses. Our data also demonstrate an incremental increase in the lens c-crystallin peak fraction with the aB-R120G mutation, suggesting that aBcrystallin may affect the expression of c-crystallin. Our results appear to corroborate a previous report indicating that acrystallin binds to specific regions of DNA in mouse cD/E crystallin genes. We found no evidence to confirm that the increase in c-crystallin is because of an increase in a- orbcrystallin fragments. Whether the increase is caused by increased expression or decreased degradation of c-crystallin in the heterozygous and homozygous aB-R120G mutant lenses remains to be determined. A small increase in c-crystallin by gel permeation 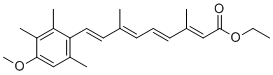 chromatography has been reported in another mouse model for cataracts. Additionally, the mutant mice accumulated aB-crystallinvimentin aggregates in lens cells and aB-crystallin-desmin aggregates in muscle cells. The mechanism of lens opacification likely involves a change in the interaction between vimentin and aB-crystallin. In heterozygous lenses, the interaction between vimentin and aB-crystallin was elevated, even in the absence of significant opacification. This increased interaction and aggregate formation were also observed in the lens epithelial zone of the mutant lenses by immunofluorescence analysis, consistent with published studies using cultured cells. These results indicate that the interaction between the intermediate filament protein vimentin and aB-crystallin may be a precursor to the development of opacities in the mutant lenses. Notably, the mice used in this study were of a mixed 129Sv and C57BL6 background, and the129Sv strain of mice is known to lack a lens-specific intermediate filament known as the beaded filament, although mice lacking the beaded filament do not develop significant opacities and changes in vimentin levels. It would be interesting to determine whether cataract development in aBR120G knock-in mice is altered in mouse strains that have the full complement of the beaded filaments. aB-crystallin is expressed in the cornea where it is important for corneal clarity. Consistent with this, a proportion of the R120G mutant mice developed corneal opacities. However, it is unclear why only a proportion of animals showed this effect. More of the animals are likely to develop corneal opacities as they age, and studies are in progress to assess this possibility. Patients with mutations in the aA-crystallin gene develop similar microcornea and corneal opacities, substantiating our findings in knockin mice and indicating that aB-crystallin also plays a critical role in the maintenance of corneal clarity. A small but significant fraction of the aB-R120G mutant mice had smaller eyes than wild-type littermates, although this small eye phenotype was not as prominent as in homozygous aA-R49C mutant mice. DRMs are a growing class of skeletal muscle disorders caused by mutations in desmin, aB-crystallin, Z-band alternatively spliced PDZ motif, myotilin, filamin C, and Bag3. Benzoylaconine Individuals with DRM typically develop late-onset progressive distal and proximal muscle weakness. Muscle biopsies from DRM patients have characteristic desmin inclusions. aB-R120G heterozygous and homozygous mutant mice recapitulate many of the pathologic features observed in DRM patients, including myopathy, desmin aggregates, and mitochondrial pathology. These mice will be invaluable for expanding our understanding of how protein aggregates lead to skeletal muscle dysfunction.
chromatography has been reported in another mouse model for cataracts. Additionally, the mutant mice accumulated aB-crystallinvimentin aggregates in lens cells and aB-crystallin-desmin aggregates in muscle cells. The mechanism of lens opacification likely involves a change in the interaction between vimentin and aB-crystallin. In heterozygous lenses, the interaction between vimentin and aB-crystallin was elevated, even in the absence of significant opacification. This increased interaction and aggregate formation were also observed in the lens epithelial zone of the mutant lenses by immunofluorescence analysis, consistent with published studies using cultured cells. These results indicate that the interaction between the intermediate filament protein vimentin and aB-crystallin may be a precursor to the development of opacities in the mutant lenses. Notably, the mice used in this study were of a mixed 129Sv and C57BL6 background, and the129Sv strain of mice is known to lack a lens-specific intermediate filament known as the beaded filament, although mice lacking the beaded filament do not develop significant opacities and changes in vimentin levels. It would be interesting to determine whether cataract development in aBR120G knock-in mice is altered in mouse strains that have the full complement of the beaded filaments. aB-crystallin is expressed in the cornea where it is important for corneal clarity. Consistent with this, a proportion of the R120G mutant mice developed corneal opacities. However, it is unclear why only a proportion of animals showed this effect. More of the animals are likely to develop corneal opacities as they age, and studies are in progress to assess this possibility. Patients with mutations in the aA-crystallin gene develop similar microcornea and corneal opacities, substantiating our findings in knockin mice and indicating that aB-crystallin also plays a critical role in the maintenance of corneal clarity. A small but significant fraction of the aB-R120G mutant mice had smaller eyes than wild-type littermates, although this small eye phenotype was not as prominent as in homozygous aA-R49C mutant mice. DRMs are a growing class of skeletal muscle disorders caused by mutations in desmin, aB-crystallin, Z-band alternatively spliced PDZ motif, myotilin, filamin C, and Bag3. Benzoylaconine Individuals with DRM typically develop late-onset progressive distal and proximal muscle weakness. Muscle biopsies from DRM patients have characteristic desmin inclusions. aB-R120G heterozygous and homozygous mutant mice recapitulate many of the pathologic features observed in DRM patients, including myopathy, desmin aggregates, and mitochondrial pathology. These mice will be invaluable for expanding our understanding of how protein aggregates lead to skeletal muscle dysfunction.
The ability of wild-type tau to promote alterations in the actin cytoskel use this mechanism to modulate its virulence
In another fungal species, Botrytis cinerea, which is a fungus causing losses of commercially important fruits, vegetables and vineyards worldwide, ABC-transporter upregulation was associated with drug resistance due to the use of fungicides. B. cinerea drug resistance is spreading, thus arguing against a fitness cost due to ABC-transporter upregulation. Regarding PUP1, no other homologues were found yet involved in microbial pathogenesis and therefore the exact role of the product encoded by this gene in C. glabrata pathogenesis remains an open question. LOUREIRIN-B cortical neurons and BSMC sensitive to amyloid protein were susceptible to 15d-PGJ2. 15d-PGJ2 bound specifically to the two cells, suggesting that 15d-PGJ2 played an important role in amyloidoses not only in the central nervous system but also in the peripheral tissues. The specific binding sites of 15d-PGJ2 were detected in the neuronal subcellular fractions of nuclear, cytosol and plasma membrane, but not in the Tulathromycin B microsomal fraction. 15dPGJ2 binds to the nuclear receptor, PPARc and the cytosolic protein, Ras. In peripheral tissues including nerves, chemoattractant receptor-homologous molecule expressed on Th2 cells has been identified as a type 2 receptor for PGD2, and reported to be also a membrane receptor for 15d-PGJ2. Contrary to its mRNA, little protein of DP2 has yet been detected in the central nerve. Furthermore, we ruled out the possibility that the specific binding site of 15d-PGJ2 in the plasma membrane of cortical neurons was DP2. First, few binding sites of PGD2 are detected in plasma membranes from rat cortices. Although binding sites of ?12-PGJ2 and PGJ2 are also detected in plasma membranes, those are displaced most potently by 15d-PGJ2 among PGD2 metabolites. Second, a DP2 selective agonist, 15d-PGD2 do not affect the cell number of neuronal cells and BSMC. Enolase 1 and Enolase 2 belong to a superfamily of abundantly expressed carbon-oxygen lyases known for the catalysis of 2phosphoglycerate to phosphoenolpyruvate. Ubiquitous enolase1 and neuron specific enolase 2 exist as monomers and also as dimmers on the neuronal membrane surface. Recent studies have demonstrated that enolases possess different regulatory properties from glycolysis in the brain. Enolase1 is one of the most consistently up-regulated and oxidatively modified proteins in brain of subjects of early-onset AD. Enolase1 and enolase 2 are autoantigen targets in post-streptococcal autoimmune disease of central nervous system. The anti-enolase antibodies induce neuronal apoptosis. Enolase 2 is part of neuronal PMOs, and the anti-enolase2 antibody can inhibit PMO activity on the plasma membrane. Pyruvate kinase transfers a phophate from phosphoenolpyruvate to ADP. Pyruvate kinase is also defined as the autoantigen, and its antibodies induce neuronal apoptosis. The significant increase in pyruvate kinase activity is found in frontal and temporal cortex of AD brains. Pyruvate kinase is elevated in the cortical neurons undergoing Ab-mediated apoptosis. Pyruvate kinase is co-precipitated with fAb. Biotinylated 15dPGJ2 binds to PKM1 in mesangial cells, supporting our results. Tubulin has been identified as a membrane component of synaptosomes and various plasma membranes. Both tubulin a and b have been shown to associate with the amyloid deposits of familial amyloidosis and to bind to the Ab sequence of APP. Moreover, tubulin b is retained by a monomeric Ab column, and co-precipitated with fAb. The tau protein interacts with tubulin to stabilize microtubules and promote tubulin assembly into microtubules. PGJ2 induces caspase-mediated cleavage of tau, generating Dtau, an aggregation prone form known to seed tau aggregation prior to neurofibrillary tangle formation. Hyperphosphorylation of the tau protein can result in the self-assembly of tangles of paired helical filaments and straight filaments, which are involved in the pathogenesis of AD. Biotinylated 15d-PGJ2  binds to tubulin b in mesangial cells, supporting our results.
binds to tubulin b in mesangial cells, supporting our results.