In this work, we have demonstrated that fepA was also negatively controlled, but by a TetR-type repressor. TetR proteins constitute a well-known family of transcriptional repressors. They have been extensively studied in the regulation of several genes for drug efflux systems, such as TetR and tetA in E. coli, AcrR 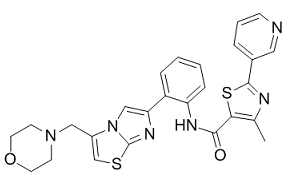 and acrAB in E. coli, AdeN and AdeIJK in Acinetobacter baumannii, or QacR and qacA/qacB in S. aureus. As previously reported for other TetR-like repressors, FepR also autoregulates expression of its own gene. As opposed to what was observed for fepR, TetR-likeencoding genes are usually divergently transcribed and are not part of an operon with the structural gene for the efflux pump. Finally, no data are available about the expression of efflux pumps during the cell cycle. For fepA, it seems to be highly expressed during the exponential phase, like most of the genes controlled by s70 factors, but further investigations are needed. In conclusion, this is the first characterization of a MATE efflux pump involved in FQ resistance in L. monocytogenes. The substrate profile appears to be narrow, including only hydrophilic FQs. Finally, we also report transcriptional regulation of the expression of a MATE family efflux pump-encoding gene through a TetR-like repressor. Similar molecular mechanisms may be involved in FQ resistance within other important gram-positive pathogens in which FepA homologs are chromosomally encoded and for which FQ are indicated. Liver ischemia reperfusion injury is a clinically relevant condition that occurs during resection surgery, Gentamycin Sulfate trauma, hypovolemic shock, or transplantation when liver is transiently deprived of oxygen and reoxygenated. These conditions result in hepatic dysfunction and failure as well as remote organ injury. The pathophysiology of liver IRI includes direct cellular damage as the result of the ischemic insult as well as delayed dysfunction and damages that result from activation of inflammatory pathways. Clinical and experimental data have established that up to 10% early graft dysfunction and higher incidence of both acute and chronic rejection are associated with IRI, and therefore, it dampens the long-term graft survival. Hepatic injuries caused by IRI are now recognized as a result of highly complex mechanisms, among which, the role of T lymphocytes has been proved of great importance and as a key mediator of IRI. These studies indicate that T lymphocyte is the key regulator in initiating and propagating the injury response. One may therefore speculate whether a reduction in T lymphocytes may reduce the incidence and severity of IR-induced complications. IL-2R-specific monoclonal antibody was used in clinics to inhibit most of the IL-2/IL-2R interaction for a considerable time, and prevented rejection in organ transplantation. It acts as an antagonist at the interleukin-2 binding site of the p55 subunit of the high affinity IL-2 receptor on the surface of the activated T lymphocytes and inhibits the binding of serum IL-2 to CD25, there by inhibiting the proliferation of activated T cells and subsequent release of cytokines. However, one of the most important conflicts is that the current interventions targeting the IL-2R through antiCD25 mAb can reduce the number and function of Treg cells, and eventually aggravate the IR injury. In the present study, we sought to elucidate whether Dimesna near-term intervention targeting the IL-2R through anti-CD25 mAb might compromise the number or function of Treg cells in the liver.
and acrAB in E. coli, AdeN and AdeIJK in Acinetobacter baumannii, or QacR and qacA/qacB in S. aureus. As previously reported for other TetR-like repressors, FepR also autoregulates expression of its own gene. As opposed to what was observed for fepR, TetR-likeencoding genes are usually divergently transcribed and are not part of an operon with the structural gene for the efflux pump. Finally, no data are available about the expression of efflux pumps during the cell cycle. For fepA, it seems to be highly expressed during the exponential phase, like most of the genes controlled by s70 factors, but further investigations are needed. In conclusion, this is the first characterization of a MATE efflux pump involved in FQ resistance in L. monocytogenes. The substrate profile appears to be narrow, including only hydrophilic FQs. Finally, we also report transcriptional regulation of the expression of a MATE family efflux pump-encoding gene through a TetR-like repressor. Similar molecular mechanisms may be involved in FQ resistance within other important gram-positive pathogens in which FepA homologs are chromosomally encoded and for which FQ are indicated. Liver ischemia reperfusion injury is a clinically relevant condition that occurs during resection surgery, Gentamycin Sulfate trauma, hypovolemic shock, or transplantation when liver is transiently deprived of oxygen and reoxygenated. These conditions result in hepatic dysfunction and failure as well as remote organ injury. The pathophysiology of liver IRI includes direct cellular damage as the result of the ischemic insult as well as delayed dysfunction and damages that result from activation of inflammatory pathways. Clinical and experimental data have established that up to 10% early graft dysfunction and higher incidence of both acute and chronic rejection are associated with IRI, and therefore, it dampens the long-term graft survival. Hepatic injuries caused by IRI are now recognized as a result of highly complex mechanisms, among which, the role of T lymphocytes has been proved of great importance and as a key mediator of IRI. These studies indicate that T lymphocyte is the key regulator in initiating and propagating the injury response. One may therefore speculate whether a reduction in T lymphocytes may reduce the incidence and severity of IR-induced complications. IL-2R-specific monoclonal antibody was used in clinics to inhibit most of the IL-2/IL-2R interaction for a considerable time, and prevented rejection in organ transplantation. It acts as an antagonist at the interleukin-2 binding site of the p55 subunit of the high affinity IL-2 receptor on the surface of the activated T lymphocytes and inhibits the binding of serum IL-2 to CD25, there by inhibiting the proliferation of activated T cells and subsequent release of cytokines. However, one of the most important conflicts is that the current interventions targeting the IL-2R through antiCD25 mAb can reduce the number and function of Treg cells, and eventually aggravate the IR injury. In the present study, we sought to elucidate whether Dimesna near-term intervention targeting the IL-2R through anti-CD25 mAb might compromise the number or function of Treg cells in the liver.
Monthly Archives: May 2019
Both bone and cartilage formation boosting the MSCs among cells with cartilage forming capacity
However, two factors influence the formation of bone tissue: the local milieu and the maintenance of stem cells in situ. Immunogenicity may be removed from freeze-dried bone allografts but cortical bone remains dense. This scenario is not conducive to permitting cells and nutrients to penetrate bone. Hence, we created many pores on the bone allograft scaffolds to permit the penetration of cells and nutrients. Fibrin glue was used in this study to maintain the position of the stem cells. Several studies have suggested that fibrin glue can be used for cell delivery because it is a biocompatible and biodegradable tissue adhesive that stabilizes seeded cells and provides an equally distributed population of cells throughout the carrier. Authors have demonstrated that fibrin glue does not inhibit the proliferation of bonemarrow MSCs. In this study, allogeneic bone began to absorb 1 month after transplantation in the experimental group. Absorption was significant 3 months after transplantation and new bone was formed at this time. The size of new bone was smaller than that of the original bone allograft scaffold, and almost all the bone allograft scaffold was CAY10505 replaced by new bone 1 year after transplantation. However, in the control group, the bone allograft was not absorbed 1 year after transplantation. The speed of the formation of new bone was slower than that observed in the experimental group. Approximately 30% of allogenic bone was replaced by new bone. It is possible that more dense bone was formed at the cortical bone, and therefore angiogenesis was slower. Resorption of allogeneic bone in the experimental group was significantly greater, and formation of new bone faster, than that seen in the control group. These findings demonstrated that the mechanism of healing of bone allografts changed when bone marrow-derived MSCs were loaded onto the scaffolds. It is possible that the addition of cells with high osteoblastic potential could enhance the bone appositional phase from the early stages of remodeling. Nevertheless, investigation of the specific underlying mechanism merits further study. However, histological analysis has shown that although almost all transplanted allogeneic bone was replaced by new bone in the experimental group, most of it was fibrous ossification. MSCs have the potential for multilineage differentiation into osteoblasts, chondrocytes, adipocytes, myocytes, cardiomyocytes, and neurons, amongst others. They can differentiate into osteoblasts in the presence of osteoinductive factors 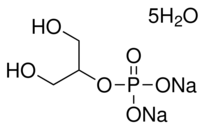 or an osteogenic environment. In this study, we used scaffolds and seed cells, and did not add Indinavir sulfate growth factors during the reconstruction of hemi-mandibular defects. Although the freeze-dried bone contain many growth factors such as BMP, the presence of growth factors in the freezedried bone was very limited. These limited growth factors could not have induced MSCs to differentiate into osteoblasts. It is possible reason that most of the “new bone” was fibrous ossification. In future studies, we will add growth factors such as BMP to assess whether more optimal results can be gained. In summary, we have demonstrated that tissue-engineered bone could be created using bone allograft scaffold-loaded autologous marrow MSCs. MSCs accelerate the speed of absorption of bone allografts and ossification. The major drawback is infection, as two beagles from control group and one beagle from experimental group had postoperative wound infections, and future studies will be needed to determine ways to reduce the rate.
or an osteogenic environment. In this study, we used scaffolds and seed cells, and did not add Indinavir sulfate growth factors during the reconstruction of hemi-mandibular defects. Although the freeze-dried bone contain many growth factors such as BMP, the presence of growth factors in the freezedried bone was very limited. These limited growth factors could not have induced MSCs to differentiate into osteoblasts. It is possible reason that most of the “new bone” was fibrous ossification. In future studies, we will add growth factors such as BMP to assess whether more optimal results can be gained. In summary, we have demonstrated that tissue-engineered bone could be created using bone allograft scaffold-loaded autologous marrow MSCs. MSCs accelerate the speed of absorption of bone allografts and ossification. The major drawback is infection, as two beagles from control group and one beagle from experimental group had postoperative wound infections, and future studies will be needed to determine ways to reduce the rate.
Alternative for multi-color imaging because it only requires a single wavelength measurement
First of all, thrombin was applied on the PC12 cell line to imitate an in vitro ICH condition, but it is Yunaconitine evident that more extensive mechanisms are related to post-hemorrhagic neuronal damage in the real clinical situation.2 It is impossible to simulate perihematomal inflammation or tissue hypoperfusion by an in vitro thrombin injury model. Considering that the PC12 cell line is from pheochromocytoma tissue, the miRNA expression level and protective effect of let7c derived from in vitro thrombin injury may be different from  the brain tissue response after ICH. However, the neuroprotective effect of AM let7c in the in vitro thrombin injury model was validated in the vivo ICH model, in which apoptotic cell death was reduced by promoting IGF1R signaling. Only the intranasal delivery of AM let7c was studied in this study, although there is a chance that other administration routes such as intracerebral injection could be more efficient. Future studies regarding dose response, time window, as well as the route of administration will help to strengthen the feasibility of miRNA modulation strategy in ICH. In conclusion, we demonstrated a distinct miRNA expression pattern after ICH and its modulation can have therapeutic potential. The let7c antagomir reduced cell death and inflammation, and enhanced neurological recovery by activating the IGF1R pro-survival pathway. This suggests blocking let7c might be a potential therapeutic target in ICH. A variety of hormones or neurotransmitters, such as adrenaline, dopamine, and prostaglandins, stimulate specific G protein-coupled receptors and activate or suppress adenylate cyclase, leading to an increase or decrease in cAMP. Cyclic AMP directly binds and activates at least three effectors, protein kinase A, exchange protein directly activated by cAMP, and cyclic nucleotidegated channels, and mediates various cellular functions via distinct pathways. Because some cAMP effector proteins are localized only in specific subcellular components, spatial and temporal cAMP dynamics are crucial for regulation of various cellular functions. For investigation of spatial and temporal dynamics of intracellular messenger molecules, such as Ca2+, cAMP and cGMP, two types of genetically-encoded fluorescent indicators have been developed. These indicators are Fo��rster resonance energy transfer -based ratiometric indicators and single fluorescent protein -based intensiometric indicators. FRET-based ratiometric indicators enable monitoring of dynamics of intracellular molecules by a ratio of fluorescence intensity change at two different wavelengths. FP-based intensiometric indicators enable monitoring of dynamics of intracellular molecules by a change in fluorescence intensity of a single wavelength. To monitor cAMP dynamics in cells, several FRET-based cAMP sensors have been CAY10505 generated. The FRET-based cAMP indicators contain a cAMP binding domain in the effector molecules. These are tagged with a pair of FPs, such as cyan FP and yellow FP, for detecting a change in FRET signal induced by binding of cAMP to the domains. Although live-cell imaging using FRET-based cAMP indicators can visualize spatial and temporal dynamics of cAMP, two different emission wavelengths are required for measurement. This limits available wavelengths for multi-color imaging in combination with other signaling molecule indicators. A single FP-based intensiometric indicator is a potential.
the brain tissue response after ICH. However, the neuroprotective effect of AM let7c in the in vitro thrombin injury model was validated in the vivo ICH model, in which apoptotic cell death was reduced by promoting IGF1R signaling. Only the intranasal delivery of AM let7c was studied in this study, although there is a chance that other administration routes such as intracerebral injection could be more efficient. Future studies regarding dose response, time window, as well as the route of administration will help to strengthen the feasibility of miRNA modulation strategy in ICH. In conclusion, we demonstrated a distinct miRNA expression pattern after ICH and its modulation can have therapeutic potential. The let7c antagomir reduced cell death and inflammation, and enhanced neurological recovery by activating the IGF1R pro-survival pathway. This suggests blocking let7c might be a potential therapeutic target in ICH. A variety of hormones or neurotransmitters, such as adrenaline, dopamine, and prostaglandins, stimulate specific G protein-coupled receptors and activate or suppress adenylate cyclase, leading to an increase or decrease in cAMP. Cyclic AMP directly binds and activates at least three effectors, protein kinase A, exchange protein directly activated by cAMP, and cyclic nucleotidegated channels, and mediates various cellular functions via distinct pathways. Because some cAMP effector proteins are localized only in specific subcellular components, spatial and temporal cAMP dynamics are crucial for regulation of various cellular functions. For investigation of spatial and temporal dynamics of intracellular messenger molecules, such as Ca2+, cAMP and cGMP, two types of genetically-encoded fluorescent indicators have been developed. These indicators are Fo��rster resonance energy transfer -based ratiometric indicators and single fluorescent protein -based intensiometric indicators. FRET-based ratiometric indicators enable monitoring of dynamics of intracellular molecules by a ratio of fluorescence intensity change at two different wavelengths. FP-based intensiometric indicators enable monitoring of dynamics of intracellular molecules by a change in fluorescence intensity of a single wavelength. To monitor cAMP dynamics in cells, several FRET-based cAMP sensors have been CAY10505 generated. The FRET-based cAMP indicators contain a cAMP binding domain in the effector molecules. These are tagged with a pair of FPs, such as cyan FP and yellow FP, for detecting a change in FRET signal induced by binding of cAMP to the domains. Although live-cell imaging using FRET-based cAMP indicators can visualize spatial and temporal dynamics of cAMP, two different emission wavelengths are required for measurement. This limits available wavelengths for multi-color imaging in combination with other signaling molecule indicators. A single FP-based intensiometric indicator is a potential.