However, two factors influence the formation of bone tissue: the local milieu and the maintenance of stem cells in situ. Immunogenicity may be removed from freeze-dried bone allografts but cortical bone remains dense. This scenario is not conducive to permitting cells and nutrients to penetrate bone. Hence, we created many pores on the bone allograft scaffolds to permit the penetration of cells and nutrients. Fibrin glue was used in this study to maintain the position of the stem cells. Several studies have suggested that fibrin glue can be used for cell delivery because it is a biocompatible and biodegradable tissue adhesive that stabilizes seeded cells and provides an equally distributed population of cells throughout the carrier. Authors have demonstrated that fibrin glue does not inhibit the proliferation of bonemarrow MSCs. In this study, allogeneic bone began to absorb 1 month after transplantation in the experimental group. Absorption was significant 3 months after transplantation and new bone was formed at this time. The size of new bone was smaller than that of the original bone allograft scaffold, and almost all the bone allograft scaffold was CAY10505 replaced by new bone 1 year after transplantation. However, in the control group, the bone allograft was not absorbed 1 year after transplantation. The speed of the formation of new bone was slower than that observed in the experimental group. Approximately 30% of allogenic bone was replaced by new bone. It is possible that more dense bone was formed at the cortical bone, and therefore angiogenesis was slower. Resorption of allogeneic bone in the experimental group was significantly greater, and formation of new bone faster, than that seen in the control group. These findings demonstrated that the mechanism of healing of bone allografts changed when bone marrow-derived MSCs were loaded onto the scaffolds. It is possible that the addition of cells with high osteoblastic potential could enhance the bone appositional phase from the early stages of remodeling. Nevertheless, investigation of the specific underlying mechanism merits further study. However, histological analysis has shown that although almost all transplanted allogeneic bone was replaced by new bone in the experimental group, most of it was fibrous ossification. MSCs have the potential for multilineage differentiation into osteoblasts, chondrocytes, adipocytes, myocytes, cardiomyocytes, and neurons, amongst others. They can differentiate into osteoblasts in the presence of osteoinductive factors 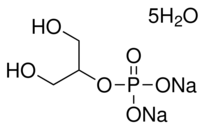 or an osteogenic environment. In this study, we used scaffolds and seed cells, and did not add Indinavir sulfate growth factors during the reconstruction of hemi-mandibular defects. Although the freeze-dried bone contain many growth factors such as BMP, the presence of growth factors in the freezedried bone was very limited. These limited growth factors could not have induced MSCs to differentiate into osteoblasts. It is possible reason that most of the “new bone” was fibrous ossification. In future studies, we will add growth factors such as BMP to assess whether more optimal results can be gained. In summary, we have demonstrated that tissue-engineered bone could be created using bone allograft scaffold-loaded autologous marrow MSCs. MSCs accelerate the speed of absorption of bone allografts and ossification. The major drawback is infection, as two beagles from control group and one beagle from experimental group had postoperative wound infections, and future studies will be needed to determine ways to reduce the rate.
or an osteogenic environment. In this study, we used scaffolds and seed cells, and did not add Indinavir sulfate growth factors during the reconstruction of hemi-mandibular defects. Although the freeze-dried bone contain many growth factors such as BMP, the presence of growth factors in the freezedried bone was very limited. These limited growth factors could not have induced MSCs to differentiate into osteoblasts. It is possible reason that most of the “new bone” was fibrous ossification. In future studies, we will add growth factors such as BMP to assess whether more optimal results can be gained. In summary, we have demonstrated that tissue-engineered bone could be created using bone allograft scaffold-loaded autologous marrow MSCs. MSCs accelerate the speed of absorption of bone allografts and ossification. The major drawback is infection, as two beagles from control group and one beagle from experimental group had postoperative wound infections, and future studies will be needed to determine ways to reduce the rate.
Both bone and cartilage formation boosting the MSCs among cells with cartilage forming capacity
Leave a reply