TLR5 is expressed highly in some cancer cells, but is not expressed on mouse macrophages and conventional dendritic cells. TLR5 recognizes flagellin and initiates a signaling cascade through recruitment of MyD88 and activation of NF-kB. Recently, we and other groups determined that among TLR ligands, only the TLR5 ligand flagellin can induce TLR signaling in breast cancer cells. Triggering of TLR5 in cancer cells inhibits cancer cell proliferation and elicits strong antitumor activity. TLR5 signaling also exhibits radioprotective activity and improves the radiation efficacy of tumor cells in radiotherapy. However, TLR5 signaling in gastric cancer exhibits the opposite effect. The reason for these different outcomes of TLR5 signaling in different cancers is not clear. In this study, we focus on the role of MAP1S in TLR5-induced suppression of breast cancer. MAP1S is a recently identified adaptor protein of autophagic processes, which participates in microtubular coordination and regulates autophagy to suppress tumorigenesis. We observed that MAP1S levels were upregulated in response to flagellin Tubuloside-A treatment in human breast carcinomas 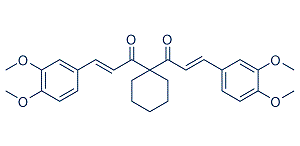 and MAP1S regulated cytokine expression induced by TLR5 signaling. Remarkably, MAP1S was associated with inhibition of cell proliferation and migration of flagellin-treated breast cancer cells. In addition, flagellin-induced elevation of MAP1S expression was involved in inhibitory feedback regulation of TLR5 signaling-induced late stage inflammation through the degradation of MyD88. Increasing evidence suggests that TLR5 signaling may play a role in tumorigenesis. Our previous results also Tenuifoliside-C showed that activation of TLR5 by flagellin elicited strong antitumor activity in breast cancer cells. In this study, we further investigated the underlying antitumor mechanisms of TLR5 signaling in breast cancer cells by examining the function of MAP1S. MAP1S is an important autophagic adaptor and is linked with suppression of tumorigenesis through the regulation of autophagy. We found that MAP1S levels are elevated in response to flagellin stimulation in MCF-7 cells, but it does not responded to LPS treatment. Flagellin-treated MCF-7 cells exhibited increases in the number of LC3 punctate foci, an autophagy marker. MCF-7 cells transfected with CD4/TLR4 or MAP1S alone showed few LC3 foci, while transfection of both plasmids induced larger numbers of foci, suggesting that MAP1S enhances TLR signalinginduced autophagy. Furthermore, knockdown of MAP1S dramatically decreased expression of the cytokines IL-8 and TNF-a and decreased NF-kB activity induced by TLR5 signaling. Taken together, these observations indicate that MAP1S is an autophagic regulator involved in TLR5 signaling in breast cancer cells. Consistent with our previous reported results, we demonstrated that flagellin suppressed proliferation of breast cancer cells. Furthermore, the whole-cell MALDI-TOF MS assay showed that flagellin inhibited MCF-7 cell state of activation globally. We also found MAP1S played a critical role in tumor suppression induced by flagellin treatment. Knockdown of MAP1S almost completely abrogated the inhibition of tumor growth and migration by flagellin treatment, which is consistent with a previous report showing that MAP1S deficient mice frequently develop tumors. We observed G1/S arrest, a significant decrease of cell cyclin protein CyclinD1 and increased p27 levels in MCF-7/shCtrl cells upon flagellin treatment, while there were no obvious changes in CyclinD1 and p27 levels in flagellin-treated MCF-7/shMAP1S cells. These results indicated MAP1S played an important role in antitumor activity of flagellin/ TLR5 signaling in MCF-7 cells. MAP1S enhances autophagic activity and is induced by stress exposure, indicating autophagic activation.
and MAP1S regulated cytokine expression induced by TLR5 signaling. Remarkably, MAP1S was associated with inhibition of cell proliferation and migration of flagellin-treated breast cancer cells. In addition, flagellin-induced elevation of MAP1S expression was involved in inhibitory feedback regulation of TLR5 signaling-induced late stage inflammation through the degradation of MyD88. Increasing evidence suggests that TLR5 signaling may play a role in tumorigenesis. Our previous results also Tenuifoliside-C showed that activation of TLR5 by flagellin elicited strong antitumor activity in breast cancer cells. In this study, we further investigated the underlying antitumor mechanisms of TLR5 signaling in breast cancer cells by examining the function of MAP1S. MAP1S is an important autophagic adaptor and is linked with suppression of tumorigenesis through the regulation of autophagy. We found that MAP1S levels are elevated in response to flagellin stimulation in MCF-7 cells, but it does not responded to LPS treatment. Flagellin-treated MCF-7 cells exhibited increases in the number of LC3 punctate foci, an autophagy marker. MCF-7 cells transfected with CD4/TLR4 or MAP1S alone showed few LC3 foci, while transfection of both plasmids induced larger numbers of foci, suggesting that MAP1S enhances TLR signalinginduced autophagy. Furthermore, knockdown of MAP1S dramatically decreased expression of the cytokines IL-8 and TNF-a and decreased NF-kB activity induced by TLR5 signaling. Taken together, these observations indicate that MAP1S is an autophagic regulator involved in TLR5 signaling in breast cancer cells. Consistent with our previous reported results, we demonstrated that flagellin suppressed proliferation of breast cancer cells. Furthermore, the whole-cell MALDI-TOF MS assay showed that flagellin inhibited MCF-7 cell state of activation globally. We also found MAP1S played a critical role in tumor suppression induced by flagellin treatment. Knockdown of MAP1S almost completely abrogated the inhibition of tumor growth and migration by flagellin treatment, which is consistent with a previous report showing that MAP1S deficient mice frequently develop tumors. We observed G1/S arrest, a significant decrease of cell cyclin protein CyclinD1 and increased p27 levels in MCF-7/shCtrl cells upon flagellin treatment, while there were no obvious changes in CyclinD1 and p27 levels in flagellin-treated MCF-7/shMAP1S cells. These results indicated MAP1S played an important role in antitumor activity of flagellin/ TLR5 signaling in MCF-7 cells. MAP1S enhances autophagic activity and is induced by stress exposure, indicating autophagic activation.
In agreement with this study we observed that elevated MAP1S levels were activity
Leave a reply