We applied these CX-4945 PKC inhibitor assays to measure sporulation efficiency in response to treatment with 446 drugs that have been tested in human clinical trials for a wide variety of therapeutic indications. Out of these, 12 were identified that inhibited 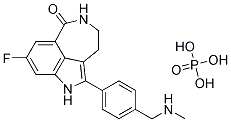 meiotic development, but not vegetative growth. Strikingly, these sporulation-specific inhibitors were structurally related to a class of compounds called cationic amphiphilic drugs, or CADs. Members of this class are weak bases with lipophilic properties, and tend to accumulate in acidic intracellular compartments such as lysosomes. Once inside the acidic milieu of the lysosome, the molecules NVP-BEZ235 PI3K inhibitor becomeprotonated, can no longer permeate the membrane and get trapped inside the organelle, a phenomenon referred to as lysosomotropism. Ultimately, the excess accumulation of CADs can give rise to a lysosomal storage disorder, called phospholipidosis. Hallmarks of phospholipidosis are the formation of multilamellar vesicles that can lead to the disruption of organelle integrity and an alteration of phospholipid metabolism. Recent work demonstrated that the antidepressant CAD sertraline evokes phenotypes in yeast that resemble those of phospholipidosis. Cationic amphiphiles have also been shown to interfere with the process of autophagy. During autophagy, cytoplasmic cargo is captured into autophagosomes, a double membraned vesicle, followed by fusion of the autophagosome with the lysosome/vacuole to form an autolysosome where the captured material is degraded. The antimalarial drug chloroquine, a CAD, has been shown to accumulate inside autophagic vacuoles and to increase the intralysosomal pH. This inhibits the acid-dependent degradation of autophagosome content and results in the accumulation of autophagic vesicles that cannot be cleared from the cytoplasm. Similarly, yeast cells treated with sertraline, appeared to contain large inclusions of incompletely digested autophagosomes and vacuoles exhibiting increased electron-transparency, suggesting a loss of vacuole acidity and/or impaired delivery of vacuolar hydrolases. In yeast the limitation for any of the essential nutrients can trigger autophagy, with nitrogen limitation displaying the strongest stimulus. In the absence of external nitrogen sources, yeast defective in autophagy experience a strong depletion of internal amino acids, which precludes the synthesis of proteins important for surviving nitrogen starvation and can result in accelerated cell death. Autophagy therefore provides the primary source of nitrogen under starvation condition. This is presumably also the case during sporulation, a process that is induced in yeast when external nutrients are lacking. Indeed, several studies have demonstrated that autophagy is essential to sporulating cells. Several observations made in the present study support a model in which TA could inhibit sporulation by interfering with autophagy. First, the chemical-genomic screen with the homozygous deletion collection identified autophagy-related mutants as hypersensitive to TA. Interestingly, some of the genes that were identified in this screen are involved in autophagosome formation, such as ATG2, ATG9, orATG18. A possible interpretation of our screening data is therefore, that a non-essential pathway that functions in parallel to autophagosome formation, for example its fusion with the lysosome, is affected by TA. Second, transcription of genes involved in amino acid metabolism and transport were found to be up-regulated in the presence of the drug, suggesting that the cells are experiencing a lack of internal amino acids.
meiotic development, but not vegetative growth. Strikingly, these sporulation-specific inhibitors were structurally related to a class of compounds called cationic amphiphilic drugs, or CADs. Members of this class are weak bases with lipophilic properties, and tend to accumulate in acidic intracellular compartments such as lysosomes. Once inside the acidic milieu of the lysosome, the molecules NVP-BEZ235 PI3K inhibitor becomeprotonated, can no longer permeate the membrane and get trapped inside the organelle, a phenomenon referred to as lysosomotropism. Ultimately, the excess accumulation of CADs can give rise to a lysosomal storage disorder, called phospholipidosis. Hallmarks of phospholipidosis are the formation of multilamellar vesicles that can lead to the disruption of organelle integrity and an alteration of phospholipid metabolism. Recent work demonstrated that the antidepressant CAD sertraline evokes phenotypes in yeast that resemble those of phospholipidosis. Cationic amphiphiles have also been shown to interfere with the process of autophagy. During autophagy, cytoplasmic cargo is captured into autophagosomes, a double membraned vesicle, followed by fusion of the autophagosome with the lysosome/vacuole to form an autolysosome where the captured material is degraded. The antimalarial drug chloroquine, a CAD, has been shown to accumulate inside autophagic vacuoles and to increase the intralysosomal pH. This inhibits the acid-dependent degradation of autophagosome content and results in the accumulation of autophagic vesicles that cannot be cleared from the cytoplasm. Similarly, yeast cells treated with sertraline, appeared to contain large inclusions of incompletely digested autophagosomes and vacuoles exhibiting increased electron-transparency, suggesting a loss of vacuole acidity and/or impaired delivery of vacuolar hydrolases. In yeast the limitation for any of the essential nutrients can trigger autophagy, with nitrogen limitation displaying the strongest stimulus. In the absence of external nitrogen sources, yeast defective in autophagy experience a strong depletion of internal amino acids, which precludes the synthesis of proteins important for surviving nitrogen starvation and can result in accelerated cell death. Autophagy therefore provides the primary source of nitrogen under starvation condition. This is presumably also the case during sporulation, a process that is induced in yeast when external nutrients are lacking. Indeed, several studies have demonstrated that autophagy is essential to sporulating cells. Several observations made in the present study support a model in which TA could inhibit sporulation by interfering with autophagy. First, the chemical-genomic screen with the homozygous deletion collection identified autophagy-related mutants as hypersensitive to TA. Interestingly, some of the genes that were identified in this screen are involved in autophagosome formation, such as ATG2, ATG9, orATG18. A possible interpretation of our screening data is therefore, that a non-essential pathway that functions in parallel to autophagosome formation, for example its fusion with the lysosome, is affected by TA. Second, transcription of genes involved in amino acid metabolism and transport were found to be up-regulated in the presence of the drug, suggesting that the cells are experiencing a lack of internal amino acids.
It is tempting to speculate that the granular structures that were observed in TA-treated sporulating yeastare undigested autophagosomes
Leave a reply