The therapeutic effect of bortezomib and carfilzomib as anticancer drugs is generally considered to be through alteration of protein turnover. However, these drugs produce a rapid and dramatic change in the cellular peptidome, increasing the levels of some peptides and decreasing the levels of other peptides. If these peptides are biologically active, the changes in peptide levels could contribute to the physiological effects of the drugs. Several studies have shown that intracellular peptides can influence signal transduction pathways. Many other studies have shown that synthetic peptides of 10�C20 amino acids can perturb a number of processes within the cell. Therefore, it is possible that the therapeutic and/or side effects of bortezomib and carfilzomib are mediated in part through the changes in the cellular peptidome. Resistance to LY2157299 antibiotics has become increasingly common among bacterial pathogens over the past few decades. For example, our resources to treat infections with extensively drugresistant Mycobacterium tuberculosis are extremely limited and require a therapy based on a combination of different classes of antibiotics. The emerging class of antibiotic-resistant bacteria, the carbapenem-resistant Enterobacteriaceae, which kills almost half of infected patients, is also a major health concern as all antibiotics currently available are ineffective. Despite this trend, the antibacterial drug development pipeline flow is low and the number of new drugs available is rapidly decreasing. With notable increases in antibiotic resistance, the aging of the population and the fact that infectious diseases remain one of the leading causes of death worldwide, there is an urgent need for additional and diverse therapeutic strategies to treat infections. Promising approaches for treatment of infectious diseases have been emerging. These include anti-virulence agents that target bacterial virulence determinants, or host-directed therapies, such as immunomodulatory drugs that enhance host immunity to promote more effective anti-microbial attack. Hosttargeted approaches possess major advantages compared to classic antibiotics that aim to kill or reduce bacterial growth, such as reducing selection for resistance genotypes, as there is less or no selective pressure directly imposed on the pathogen. TWS119 Moreover, stimulation of the innate immune response may provide broadspectrum protection against a range of pathogenic microorganisms, including bacteria, virus and parasites. Host-directed therapies may be used as adjunct treatments to synergize with commonly used anti-microbial drugs and may also allow diversification of therapeutic strategies currently available. Protein ubiquitination is a reversible post-translational modification that regulates diverse cellular processes, 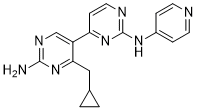 such as DNA repair, cell division, signaling, protein degradation and notably, innate immune function. Ubiquitination occurs by covalent attachment of an,8.5 kDa ubiquitin molecule to a lysine residue in the target protein by the sequential action of three enzymes; a ubiquitin-activating enzyme, a ubiquitin-conjugating enzyme and a ubiquitin-ligase enzyme. Ubiquitin is removed from proteins by deubiquitinases by proteolysis. The human genome encodes over 100 proteins that possess putative DUB activity but physiological substrates of these proteins remain poorly defined for most. DUB enzymes have established roles in a broad spectrum of diseases such as cancer, viral infection and neurodegenerative disorders. Although the function of most DUBs in immune regulation is not known, a few are key players in the modulation of innate immunity and inflammation. For example, the deubiquitinases, A20 and CYLD, control NF-kB signaling, a critical pathway in immunity and cell survival.
such as DNA repair, cell division, signaling, protein degradation and notably, innate immune function. Ubiquitination occurs by covalent attachment of an,8.5 kDa ubiquitin molecule to a lysine residue in the target protein by the sequential action of three enzymes; a ubiquitin-activating enzyme, a ubiquitin-conjugating enzyme and a ubiquitin-ligase enzyme. Ubiquitin is removed from proteins by deubiquitinases by proteolysis. The human genome encodes over 100 proteins that possess putative DUB activity but physiological substrates of these proteins remain poorly defined for most. DUB enzymes have established roles in a broad spectrum of diseases such as cancer, viral infection and neurodegenerative disorders. Although the function of most DUBs in immune regulation is not known, a few are key players in the modulation of innate immunity and inflammation. For example, the deubiquitinases, A20 and CYLD, control NF-kB signaling, a critical pathway in immunity and cell survival.
Control of ubiquitination also plays an established role in targeting for the altered levels of intracellular peptides observed
Leave a reply