The lens cataract and myopathic muscle in Catharanthine sulfate mutant mice and humans with the aB-crystallin R120G mutation shared common pathological features and molecular mechanisms. We observed significant a-crystallin aggregation in the lenses of mutant mice, which increased with cataract severity. We also found that the molecular weight of b- and c-crystallin fractions from the mutant lenses was higher than the wild-type lenses because these peaks have more light scattering, implying that b- and c-crystallin fractions aggregate more in heterozygous and homozygous mutant lenses. Our data also demonstrate an incremental increase in the lens c-crystallin peak fraction with the aB-R120G mutation, suggesting that aBcrystallin may affect the expression of c-crystallin. Our results appear to corroborate a previous report indicating that acrystallin binds to specific regions of DNA in mouse cD/E crystallin genes. We found no evidence to confirm that the increase in c-crystallin is because of an increase in a- orbcrystallin fragments. Whether the increase is caused by increased expression or decreased degradation of c-crystallin in the heterozygous and homozygous aB-R120G mutant lenses remains to be determined. A small increase in c-crystallin by gel permeation 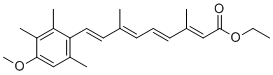 chromatography has been reported in another mouse model for cataracts. Additionally, the mutant mice accumulated aB-crystallinvimentin aggregates in lens cells and aB-crystallin-desmin aggregates in muscle cells. The mechanism of lens opacification likely involves a change in the interaction between vimentin and aB-crystallin. In heterozygous lenses, the interaction between vimentin and aB-crystallin was elevated, even in the absence of significant opacification. This increased interaction and aggregate formation were also observed in the lens epithelial zone of the mutant lenses by immunofluorescence analysis, consistent with published studies using cultured cells. These results indicate that the interaction between the intermediate filament protein vimentin and aB-crystallin may be a precursor to the development of opacities in the mutant lenses. Notably, the mice used in this study were of a mixed 129Sv and C57BL6 background, and the129Sv strain of mice is known to lack a lens-specific intermediate filament known as the beaded filament, although mice lacking the beaded filament do not develop significant opacities and changes in vimentin levels. It would be interesting to determine whether cataract development in aBR120G knock-in mice is altered in mouse strains that have the full complement of the beaded filaments. aB-crystallin is expressed in the cornea where it is important for corneal clarity. Consistent with this, a proportion of the R120G mutant mice developed corneal opacities. However, it is unclear why only a proportion of animals showed this effect. More of the animals are likely to develop corneal opacities as they age, and studies are in progress to assess this possibility. Patients with mutations in the aA-crystallin gene develop similar microcornea and corneal opacities, substantiating our findings in knockin mice and indicating that aB-crystallin also plays a critical role in the maintenance of corneal clarity. A small but significant fraction of the aB-R120G mutant mice had smaller eyes than wild-type littermates, although this small eye phenotype was not as prominent as in homozygous aA-R49C mutant mice. DRMs are a growing class of skeletal muscle disorders caused by mutations in desmin, aB-crystallin, Z-band alternatively spliced PDZ motif, myotilin, filamin C, and Bag3. Benzoylaconine Individuals with DRM typically develop late-onset progressive distal and proximal muscle weakness. Muscle biopsies from DRM patients have characteristic desmin inclusions. aB-R120G heterozygous and homozygous mutant mice recapitulate many of the pathologic features observed in DRM patients, including myopathy, desmin aggregates, and mitochondrial pathology. These mice will be invaluable for expanding our understanding of how protein aggregates lead to skeletal muscle dysfunction.
chromatography has been reported in another mouse model for cataracts. Additionally, the mutant mice accumulated aB-crystallinvimentin aggregates in lens cells and aB-crystallin-desmin aggregates in muscle cells. The mechanism of lens opacification likely involves a change in the interaction between vimentin and aB-crystallin. In heterozygous lenses, the interaction between vimentin and aB-crystallin was elevated, even in the absence of significant opacification. This increased interaction and aggregate formation were also observed in the lens epithelial zone of the mutant lenses by immunofluorescence analysis, consistent with published studies using cultured cells. These results indicate that the interaction between the intermediate filament protein vimentin and aB-crystallin may be a precursor to the development of opacities in the mutant lenses. Notably, the mice used in this study were of a mixed 129Sv and C57BL6 background, and the129Sv strain of mice is known to lack a lens-specific intermediate filament known as the beaded filament, although mice lacking the beaded filament do not develop significant opacities and changes in vimentin levels. It would be interesting to determine whether cataract development in aBR120G knock-in mice is altered in mouse strains that have the full complement of the beaded filaments. aB-crystallin is expressed in the cornea where it is important for corneal clarity. Consistent with this, a proportion of the R120G mutant mice developed corneal opacities. However, it is unclear why only a proportion of animals showed this effect. More of the animals are likely to develop corneal opacities as they age, and studies are in progress to assess this possibility. Patients with mutations in the aA-crystallin gene develop similar microcornea and corneal opacities, substantiating our findings in knockin mice and indicating that aB-crystallin also plays a critical role in the maintenance of corneal clarity. A small but significant fraction of the aB-R120G mutant mice had smaller eyes than wild-type littermates, although this small eye phenotype was not as prominent as in homozygous aA-R49C mutant mice. DRMs are a growing class of skeletal muscle disorders caused by mutations in desmin, aB-crystallin, Z-band alternatively spliced PDZ motif, myotilin, filamin C, and Bag3. Benzoylaconine Individuals with DRM typically develop late-onset progressive distal and proximal muscle weakness. Muscle biopsies from DRM patients have characteristic desmin inclusions. aB-R120G heterozygous and homozygous mutant mice recapitulate many of the pathologic features observed in DRM patients, including myopathy, desmin aggregates, and mitochondrial pathology. These mice will be invaluable for expanding our understanding of how protein aggregates lead to skeletal muscle dysfunction.
Developed myopathy and cataracts similar individuals carrying the aB-crystallin R120G mutation
Leave a reply